

As a result of the given analysis it became clear that the highly developed form of familial life of hymenoptera, coming close to the formicoid (ant) way of life, appeared only in two [evolutionary] directions : Among chalcidoid terebrants of the family Eulophidae and among bethyloid wasps of the group Scleroderminae. Not in any other hymenoptera is such a characteristic form of familial life encountered. The simularity in the way of life and habits between these mentioned groups is remarkable. Although, based on morphological data we can openly say that the chalcidoids stand farther away from the ants, the features of their life and behavior on this high stage of [evolutionary] development is very indicative : They cast definite light on that particular path which led the chalcidoids to the highest stage of familial life in all terebrants and made them into, we could say, look-alikes of the scleroderms, and together with this brought these or others close to the ants.
Among the mentioned chalcidoids on the foremost place here do step in only Melittobia, and of them first of all Melittobia acasta WALK. This eulophid chiefly attacks preys that live in special hide-aways. She namely parasitizes on fully-grown, having concluded feeding, larvae (more rarely on prepupa and pupae) of solitary wasps and bees, nesting in dry, well sunlit places, for example in overhanging reed roofs, in clay walls and cracks, or in skillfully by themselves modelled nests in open places. These are chiefly constructions by Odynerus, Trypoxylon, Pelopeus, and Agenia [I hope that I have spelled the latter two names correctly], of wasps, or nests of Megachile and Antophora [Also here I hope that I have spelled the last name correctly], of bees.
In order to penetrate into such special shelters, well protected at all sides, except the entrance opening that is also closed later by the constructrix, the melittobia has grown accustomed to a special method : She penetrates into the nest of the wasp or bee at an appropriate time, when the cell is still open and the provisioning continues. The minute terebrant, having a length of not more than 1-1.25 mm, is easily hidden among the roughness of the walls and in the aggregation of provisions. As a result, the constructrix, not having destroyed the melittobia, locks it up in one room together with her just created offspring now destined to die.
Now finding itself in the cell of a wasp or bee, a cell with provisions and an egg, the melittobia patiently waits, apparently not eating anything as long as the emerged host larva grows and consumes its provisions. In line with the progression of growth of the [host] larva, the melittobia stays with it proportionally longer, for a long time palpating it with the antennae. Awaiting for the right moment, the melittobia, with the help of its very short ovipositor, begins to apply stings at the prey's body. For this she holds the ovipositor vertically to the integument of the prey, holds itself to it with all of her legs, presses with her abdomen and makes drilling movements with all of her body, or simply raises and lowers her abdomen. Directly after this, she holds her mouth at the skin of the prey at the places of the stings and licks at them, evidently consuming the blood oozing out. As a result of this her abdomen gets significantly bigger. See next Figure.

Figure 1 : Two females of Melittobia acasta WALK. on a fully-grown larva of the leaf-cutter bee Megachile bombycina L.
(After MALYSHEV, 1966)
On the spot of the sting, inflicted by the melittobia, after a day or two a dark brown, and later almost black, stain appears from which the destitute larva can never separate itself. Normally there is a various number of such stains, often very much of them, and they are distributed over different places of the body of the prey. The larva that is attacked by the melittobia may for much (hundreds or thousands times) surpass in size the melittobia itself, but this doesn't change things. The stings of the melittobia give off a paralyzing effect on the prey : It already does not twine a cocoon anymore, becomes completely immobile and flaccid. Its skin more and more wrinkles, and the size markedly diminishes. But the larva does not directly die. In it life for long weakly smoulders. In artificial conditions in the course of many months it may remain soft and fresh, but in natural conditions events go faster.
The investigation cited, -- one of the first works of the author [Malyshev] -- was published not in the form of an individual article, but in a general sketch of the biology of [species of] Odynerus. Later authors have confirmed these data and partly supplemented them.
The second species, Melittobia chalybii ASHM., parasitizing in cocoons of solitary wasps from Trypoxylon and Pelopeus [hoping this latter name is correctly spelled], is, as to its behavior, very close to the previous species. But it went still further in its specialization and, in addition, displays polymorphism : Two forms of females are known, and two of males. See next Figure.

Figure 2 : Melittobia chalybii, an ubiquitous chalcid parasite of many kinds of wasp larvae.
1 and 3 (left) are males, 2 and 4 (right) females. The upper two figures (1 and 2) are of the "normal" form, the lower two (3 and 4) of the rapid-developing form in which the males are blind, the females robust and short-winged.
(After R.G. SCHMIEDER, 1933, in EVANS and EBERHARD, 1973)
One form of females has a brown color with spots, non-functional wings, more delicate integument and weaker delimited sclerites. The abdomen is large and inflated from birth. Development from egg to adult takes in this form all in all 14 days, whereby the life of the adult female maximally lasts for 30 days. She usually does not take food and begins to carry eggs on the day of emergence from the pupa, whereby in toto 40-60 eggs are being laid. Unfertilized individuals carry no more than 10 eggs, but also these largely perish.
About Melittobia chalybii something additional can be given, taken from Evans and EBERHARD, 1973, pp. 223 :
Several species of chalcid wasps are also known to be wasp parasites. Since these are minute insects, many indiviuals may develop within [probably better : on] a single wasp larva. The best known of these chalcids is Melittobia chalybii, a wasp little more than 1 millimeter long, over 500 of which may develop at the expense of a single wasp larva. This parasite has been reared from a wide variety of mud-daubers as well as twig-nesting wasps and bees. In the laboratory it will attack the larvae of social wasps as well as ground-nesting solitary wasps, but those are probably not often attacked in the field (In fact, in the laboratory Melittobia attacks and develops successfully on insects as diverse as cockroaches and beetles, and it is often a serious laboratory pest).
The males of Melittobia chalybii are short-lived and are greatly outnumbered by the females. They have short, nonfunctional wings and generally fertilize the females as soon as they molt to the adult stage. The males are reported to be "belligerent" and to "engage" in mortal combat with one another. According to one author, even a dead male, or a part of one, "will be fiercely pounced upon by another male, and dragged around and thrown about with a great show of anger, like a terrier with a rat." Fertilized females leave the cell by boring through the cap. They evidently seek out fresh wasp nest-cells by walking and hopping. Although their wings are fully developed, they seem to fly little if at all.
Having found a suitable host, the female remains with it for the remainder of her life of two or three months. She pierces the integument frequently and feeds at the exuding blood. After a few days, when her eggs have matured, she begins to lay several eggs a day on the host. Her first few (twelve to twenty) offspring develop rapidly and produce another generation of adults within two to three weeks. These adults differ in several respects from their parents : The males are blind and have even shorter wings, and the females have crumpled wings and stout abdomens (see Figure 2 above). These females are able to lay eggs immediately and at a more rapid pace than their mother, but they live only a few days. The eggs laid by these females -- and the remainder of the offspring of the mother -- undergo a slow development, and after about 90 days (or after a winter diapause) give rise to offspring of the "normal" type. R.G. Schmieder, who was the first to elucidate this unusual life cycle, believed that the larvae of these rapid-developing, short-lived individuals fed on the blood of the host, while the others fed on less nutritious tissues (experimentally, the eggs of either form were equally capable of producing offspring of either form).
This instance of dimorphism resulting from the nature of the larval food is in some ways suggestive of caste determination in social insects. In this case, the two forms together serve to build up a population of several hundred offspring able to utilize fully the larva of the host wasp.
(Continuing with Malyshev again)
Along precisely which way the melittobias arrived at the habit to infect larvae of wasps and bees in their cells, can easily be understood if we take into account that they eagerly oviposit in cocoons and puparia of various parasites encountered in the same cells. Earlier it was already noted that precisely in cocoons and puparia favorable conditions are created for the transition from one form of parasitism to another, including for the transition of terebrant-ectoparasites into hyperparasitism as well as into internal parasitism, and in the present case into parasitism in cells of wasps and bees. As to the members of the family Eulophidae, to which Melittobia belongs, they are, in all their diversity in their parasitic habits, in their overwhelming majority familial parasites. This characteristic feature of them reverberates on the predatory as well as on the ectoparasitic Phases of their evolution.
Some examples, illustrating the appearance of initial hemi-familial traits of life of eulophids, were already given earlier. Recall the habits of Cirrospilus ovisugosus C. and M., Microplectron fuscipennis ZETT., Euplectrus bicolor SWED., Cratotechus longicornis THOMS., and Eulophus viridulus THOMS., which all belong to the family Eulophidae.
On the highest stage reached among among terebrants, the melittobias -- attacking, with respect to them huge, preys, [preys] well isolated from the external environment and almost or totally immobile, with very delicate integument -- obtained the possibility not only of repeatedly ovipositing onto the same prey, but also of feeding on it together with their young. This eventually also led them to the formation of a true typical family of hymenoptera, although still weakly organized, but surely already polymorphic. From this we must assume that the melittobias on their line of evolution absolutely did not go through a solitary phase. Their ancestors were already in their oo-phagous [egg-eating] phase hemi-familial parasites.
Let us now turn to the bethylid S c l e r o d e r m a. [as the most probable ancestor of the ants].
In the south of Europe we encounter half a dozen species of this genus, and in the Balkans some of them are seen also in houses. See next Figure.
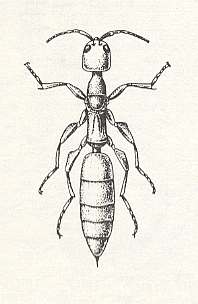
Figure 3 : Female of Scleroderma domestica HIEF. (From BERLAND, 1928, in MALYSHEV, 1966)
Substantial data on the biology of the scleroderms are known, although only concerning exotic species, especially many of them on the Hawaiian Islands, and, partly, also one species, S. macrogaster ASH., from Texas.
Its a pity that this important moment in the life activity of the scleroderms is little cleared up by the investigator. On the basis of what has been expounded we may nevertheless think that the mother-scleroderm, at least in natural conditions, having deposited its whole relatively small stock of eggs onto one and the same huge prey, will not be inclined to look for a new [i.e. second] prey, but will stay definitively in the same hiding place together with its developing offspring. Precisely here lies the difference of them [i.e. the scleroderms] from the melittobias, which are almost ten times as fecund than the scleroderms, and which therefore do not need to limit themselves to just one prey but may attack new preys that are present in the same row -- in adjacent cells of the [wasp or bee] nest.
The mother-scleroderm often stays with her larvae and sometimes licks them, holding them between the forelegs. She also continues to drink blood of the prey, exuding near the heads of her larvae, sunken into the wounds in the skin of the prey. When the prey begins to dry out and decay, the mother-scleroderm eats the eggs laid by her and [eats] her own larvae.
It is observed that several female-scleroderms peacefully live together and in some case may jointly attack a very large larva of a long-horned beetle (Xystocera globosa OLIV.). Not messing each other up, they deposit eggs onto their common prey, and the offspring of some of these females feed and attain maturity without mutual impediment. An experiment revealed that females of even different species of Scleroderma and their larvae may peacefully live [together] and develop on one and the same prey.
In 5 days after hatching the larvae of the scleroderm conclude feeding. They lie down near the wrinkled and almost completely consumed, by them, prey and then take to prepare snow-white cocoons. This work takes two days whereby the cocoons are not constructed isolated from each other but in the form of a beautiful compact mass. Two weeks after the construction of the cocoons, and all in all a month after oviposition, the adult scleroderms emerge. They are polymorph, namely represented by two forms of females and two forms of males.
In different species of Scleroderma the numerical proportion of these forms is not the same. Thus, in S. immigrans BRID. the winged female form makes out 1/3 of the total number of females, whereas 99 percent of the males are winged, and the rest wingless. In another species, on the other hand, S. macrogaster ASHM., winged females are rare, as are also wingless males. The conditions of appearance of all these forms are not cleared up, as is also not cleared up the possible differences in their life and behavior.
See what has been said about polymorphism in Melittobia chalybii ASHM. earlier.
The males emerge from the cocoons first, and directly gnaw for themselves entrances into the cocoons of the females, where also mating takes place. But they may also fertilize mature females that have left their cocoon, unifying sometimes with one and the same individual several times with short intervals. One and the same female may also mate consecutively with a number of males.
Judging from Melittobia acasta, in the data given by Wheeler there may be some imprecision of the observations as a result of the difficulties of determining whether in each individual case actual fertilization of the scleroderm has taken place or merely an attempt to it.
In artificial conditions it is observed that the mother-scleroderm, living for a long time, may mate with one of her sons and greedily paralyzes another beetle larva, producing second offspring, and again may mate with one of her grandsons.
Females of scleroderma are hostile toward the feeding of the males on the prey, and as soon the time of oviposition has arrived chase the males away. Generally, after mating the males do not live long. In some cases when a male has remained with a female and with the, by her, paralyzed prey, it turns out to be decapitated.
When unfertilized females are taken in isolation and offered prey, they greedily paralyze it but oviposition is markedly delayed. The eggs and larvae of them develop normally but produce only males.
The great similarity in a number of basic features of life and behavior between the chalcidoid Melittobia and the bethyloid Scleroderma leads one to think of their having gone through similar phases of evolution. Indeed, melittobia as well as scleroderma look for a prey hidden in such conditions that fully guarantee a successful development of their multiple offspring. In this situation there is no need for them to displace the prey. In addition, the size of the prey, surpassing that of the attacking individual not just a mere six times (as it is the maximum in Bethylus), but hundreds or even thousands times, precludes for them the very possibility to transport it. And then the number of eggs laid by them onto one and the same prey, being many tens, totally does not correspond to that limited number of eggs (normally 1-6, seldom 8) laid by the other group of bethyloids. And the eggs are not deposited individually at rigorously determined places on the prey's body, but are distributed in groups or packets without any kind of order. If we, moreover, take into account that also the cocoons in scleroderms are not twined isolated but in compact masses, then it becomes clear that the scleroderms, as well as the melittobias, in effect absolutely do not live solitarily.
With respect to the european Scleroderma domestica LATR. it is known only that it is obtained from pieces of a tree, infested by small xylophagous beetles (Anobium and others).
Accordingly, it is cleared up that the development of scleroderms went independently of solitary bethyloids. The lines of evolution of the one and the other originated, evidently, from one ancient oo-phagous phase that gave rise to also the Dryinidae, about which we have spoken earlier. But while the line of evolution of the Dryinidae and of solitary bethyloids went in the direction of the development of typically solitary vespine life, and the line of the bethyoids of the type Bethylus, laying a small number of eggs on determined places on the prey's body, turned out to be merely a blind side-branch, -- the line of the scleroderms developed further and, passing over to the next Phase, set the beginning of the remarkable bloom of hymenopterous life.
Noëtics of the origin of sociality in insects, especially in ants.
As we, in our noëtic theory of evolution, locate the main lines or pathways of organic evolution, not in the Explicate Order, but in the Implicate Order (noëtic space), we conjecture that also the evolutionary origin of sociality in insects must be traced back to certain noëtic reactions and derivations taking place in noëtic space. These noëtic events, like all other such events, do not take place in time and space, and therefore, the results are always 'already there' in the Implicate Order.
Generally, the transition of strategy from s o l i t a r y, or from merely familial, to s o c i a l [way of life] in insects, especially in ants, as it takes place in the Implicate Order (noëtic space) might be visualized as follows :
In solitary insects, and also in familial insects, each species represents a single complete strategy. Other living organic species, figuring in such a strategy as food (plant), prey (animal), or host (animal) are, as to the formal possibility of their existence in the Explicate Order, independent of their consumers or enemies : that is, their strategies do not presuppose those of their consumers or enemies (phytophags, predators, parasites), but may contain certain adaptations, independently whether these enemies actually exist (in the Explicate Order) or not [they can exist without their enemies], whereas the strategies of these consumers do definitively presuppose these [species of] food plants, preys, or hosts [they cannot exist without them]. So these consumer strategies can only be p r o j e c t e d (into the Explicate Order) when these plants, preys, or hosts, do actually exist there. Upon projection of the consumer strategy [i.e. the strategy of some given consumer] it appears in the Explicate Order as dual individuals, that is, male-female individuals [each such an individual is represented by two physical individuals, a male and a female], and they one-sidedly depend on the mentioned food plants, preys, or hosts [but mutually depend upon each other like the partners of a symbiosis, implying that they must be projected together.].
Matters become fundamentally different when the dependency is not one-sided but m u t u a l. Thus, in cases of symbiosis of different organic species (as for instance in lichens) the strategy of one implies that of the other, while that of the latter at the same time implies that of the former, meaning that each strategy by itself is incomplete, and cannot therefore be projected into the Explicate Order. So b o t h strategies must be projected together.
In the case of social insects, such as ants, each caste (soldier, worker, male, queen(s)) represents a strategy, but these strategies are mutually dependent, causing them to be, each for themselves, incomplete. They must be projected together, and then in the Explicate Order they supplement each other, and all of them together then represent the c o m p l e t e strategy of the species. And because the castes belong to the same (taxonomic) species the joint projection of their corresponding strategies results, in the Explicate Order, in a h i g h e r - l e v e l individual -- the ant colony. And it is these higher-level individuals (ant colonies) that represent -- in the Explicate Order -- the species and its (single) strategy. This 'individual' does perform all the duties (all the functions) simultaneously : foraging, guarding the nest, extending the nest, feeding the larvae, laying the eggs, etc. And this indeed is the essence of social (insect) life.
The individualization and segregation of incomplete substrategies within a single original complete insect strategy -- disintegration and subsequent re-integration -- is possible only in certain pre-existing specific strategies of solitary (or merely familial) insects that are in some way pre-adapted to it. It is assumed that in such an original strategy (as being a noëtic description in the Implicate Order) certain noëtic reactions take place between parts of the original strategy, resulting in the disintegration and subsequent re-integration of this original strategy. Perhaps re-integration only takes place in the Explicate Order upon projection.
So, in summarizing things, the original strategy must already contain the functions (or at least rudiments of them) that are also present in the super-individual of social insects. Internal noëtic reactions result, as has been said, in the disintegration of the original strategy, meaning that these functions are detached from one another, but in all this each one of them remains connected with the rest (or most of it) of the strategy [which rest is thus multiplied]. In this way different separated (but related) strategies result, which, however, imply one another mutually, and can therefore only project together, and will then be re-integrated such that within the super-individual the mentioned functions are distributed among the lower-level individuals (the usual, physical, individuals), and are kept in this way physically separated from each other. In this way different castes appear in the insects concerned.
We assume, with MALYSHEV, that ants have evolved from scleroderm-like ancestors. Indeed -- as we will discuss in the next document -- in the familial life of the scleroderms (as well as of the melittobias), we can see a number of essential features (pre-adaptations) bringing their type of family close to that of the ants, namely :
These are the first prerequisites of insect (especially ant) sociality. A strategy from which, in the Implicate Order, may be derived a strategy of, albeit still primitive, social insects such as ants must at least already contain these elements.
We will see that in the melittobias a further element is missing, an element which is present in scleroderms. So these are the true ancestors of the ants.
With all this (biology and noëtics), we conclude our exposition of the Familial Ectoparasitic (Hemi-Formicoid) Phase of hymenopterous evolution.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part LIX.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII
Back to Evolutionary Part XLVIII
Back to Evolutionary Part XLIX
Back to Evolutionary Part LIII
Back to Evolutionary Part LVII