

(Descriptions taken from ROHDENDORF, 1951, with additions and additional Figures)
Representatives of the type
The lifting lightly-veined (muscoid) type of wings are characteristic of representatives of diverse families that include a very large number of species in the recent fauna. The number of species significantly surpasses even that of the, so rich in forms, tabanoid type.
To the present type belong species of the superfamilies Conopoidea, Muscoidea, Hippoboscoidea, and the families Dolichopodidae and Clythiidae [= Platypezidae]. The total number of species is more than 16000.
To begin with, we here reproduce images of some of these representgatives :

Figure 1 : Some representatives of the Lifting Lightly-veined (muscoid) functional wing-type.
1 - Chlorops pumilionis, Yellow stem-fly, 2.5-3.5 mm. Family Chloropidae (Acalyptratae).
2- Oscinella frit, Frit-fly, 1.5-2 mm. Family Chloropidae (Acalyptratae).
3- Delia brassicae, Cabbage-fly, 5.5-7.5 mm. Family Anthomyiidae, flower-flies (Calyptratae).
6- Musca domestica, House-fly, 7-9 mm. Family Muscidae (Calyptratae).
(After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977)
While the family Dolichopodidae belongs to the Suborder Brachycera-orthorrapha, all the others (having wings of the muscoid functional type) belong to the Suborder Brachycera-cyclorrapha. Perhaps apart from the Clythiidae [= Platypezidae], all Cyclorraphic flies divide into two large sections, the Acalyptratae (fruit-flies and allies) and the Calyptratae (the house-fly and allies).
Size of the wings
Shape of the wings
Skeleton of the wing
Coverings of the wing
Functional features
Well known are the cases of breaks and disconnections of veins in the venation of the hind-wings of beetles. However, this example is not considered by me [ Rohdendorf ] and will not be compared with other cases because of the evident foremost significance of these "defects" of veins in the process of folding the wing under the elytrum ["elytra" are the protective shields originated from the fore-wings in all beetles]. The above listed cases of disconnections of veins belong to free wings and have a clearly aerodynamic significance during wing-beat.
All these insects [dragon-flies, cicadas, Hymenoptera] are characterized by high qualities of the flight-function having great significance in their life-activity. It is therefore legitimate to count the presence of breaks and disconnections in veins as an indicator of perfection of the flight-apparatus which [perfection] consists in the development of flexibility and elasticity of different parts of the wing, which undoubtedly have serious significance in the wing-beat mechanics. See next Figure.

Figure 2 : A blue-bottle of the genus Calliphora is underway to land on a lump of meat. It hurdles itself with a speed unheard of onto it and strongly bends down the wings during downbeat. For the sake of such movements the wings have a preformed snapping site at their base. This "shock landing" is not directly typical, but clearly shows how enormously manoeuverable and reactive such a fast flying insect is.
(After NACHTIGALL, Gläserne Schwingen, 1968)
Unfortunately, up untill now we must limit things to the mere observation of this fact and not try to clarify the concrete role of the vein-disconnections, which can only be done in a detailed investigation of the features of the wing-beat by special aerodynamical methods.
History and transformations of the type
Some images of three subtypes to begin with :

Figure 3 : Wing of Sciapus sp. [maybe the genus name must be spelled : "Sciopus"]. Dolichpodidae. Length 4.5 mm. Dolichopodoid subtype of the muscoid wing-type.

Figure 4 : Wing of Dolichopus sp. Dolichpodidae. Length 6 mm. Dolichopodoid subtype of the muscoid wing-type.

Figure 5 : Wing of Clythia picta Meig. Clythiidae [= Platypezidae]. Length 4.5 mm. Clythioid subtype of the muscoid wing-type.

Figure 5a : Same as previous Figure (Clythia picta). Important venational features indicated.

Figure 6 : Wing of Conops flavipes L. Conopidae. Length 8 mm. Conopoid subtype of the muscoid wing-type.

Figure 7 : Wing of Myopa sp. Conopidae. Length 4.5 mm. Conopoid subtype of the muscoid wing-type.
(All of the above Figures by ROHDENDORF, 1951)
The existing paleontological documents illuminate only the Cenozoic (Tertiary and Quaternary) faunae of the representatives of the lifting lightly-veined (muscoid) functional wing-type. Known paleogenic and neogenic diptera with wings of the muscoid type exclusively belong to families represented in the recent fauna and do not allow to clarify interrelationships and connections of the different forms of the muscoid types. Judging about the paths and directions of evolution of the muscoid wings is only possible on the basis of inspection of the most diverse recent insects, taking into account the few existing tertiary finds.
1. Dolichopodoid subtype.
Only one group of Diptera of the ancient Suborder of short-horned flies, Brachycera-orthorrapha, illustrates a special form of muscoid wings. These are the long-legged flies Dolichopodidae. See Figures 3 and 4, and 8 (next Figure).

This dolichopodoid subtype is characterized by a narrowed basiala of which the lower supporting element, the handle [= the thickened beginning] of the cubito-anal vein, is shortened and shifted apicad [i.e. shifted into the apical direction], leaving free a large central field of the basiala, surrounded by the very thick and convex handles of the Radial and Second Anal veins, carrying chaetaria. The cross-vein r-m strongly shifted toward the base of the wing. It is very short and unclear as a result of the approaching Radial and Medial veins. Very characteristic are the chief parts of the Medial and posterior Radial vein, running parallel and densely covered by microtrichia. The Costal vein has one single disconnection or thinned section immediately before the humeral cross-vein [i.e. the cross-vein near the base of the wing between the Costa and Subcosta]. The Subcostal vein is weakened, running close to, and at its end coalescing with, the Radial vein. The anterior Medial branch simple or with a curve and a rudiment of the posterior outgrowth (See Figure 3, above ). Cubital vein sharply curved, resulting in the cubital cell to be very short and rounded off at its end. Anal lobe of moderate size, sometimes very large. Alula very small. Wing-scale rudimentary, thoracic scale absent. Costalization of the wings consists of a moderate shift of the venation, leaving free of veins a large field of the membrane behind the Medial veins.
Let us [JB] consider this origin more closely [more closely, that is, than Rohdendorf did]. It cannot be represented, however, by a smooth derivation of the one from the other, but the next derivational line nevertheless shows approximately how the dolichopodoid subtype of the muscoid functional wing-type might have been originated from the microphoroid subtype of the empidoid functional wing-type.
 Microphoroid subtype of Empidoid type |
Hormopeza obliterata, Empididae. Radial Sector (RS) still 3-branched. R2 running close to R1 M1, M2, and M4 present. M3 absent. Discoidal cell still present. CuA strongly curving back on 1A, their common stalk hardly reaching wing-margin. |
 Microphoroid subtype of Empidoid type |
Microphorus velutinus, Empididae. Radius-proper running closely alongside the Subcosta (SC). Radial Sector (RS) has become 2-branched. M1, M2, and M4 present. M3 absent. Discoidal cell still present. CuA strongly curving back on 1A, their common stalk totally reduced. This form, although not smoothly so, eventually leads to muscoid wings of the dolichopodoid subtype (see next images). |
 Dolichpodoid subtype of Muscoid type |
Sciopus albifrons, Dolichopodidae. Subcosta ending up in R1. Radial Sector (RS) 2-branched. M1 and M2 having a common stalk branching off from the discoidal cell. M1 curves up towards R4+5 (or R5), as it does in many higher flies. M4 present. Discoidal cell still present. Cubital cell very small, i.e.CuA strongly curves back on 1A already at the very base of the wing. Their common stalk not reaching wing-margin. From this venation that of the following is clearly derivable. |
 Dolichpodoid subtype of Muscoid type |
Dolichopus picipes, Dolichopodidae. Subcosta ending up in R1. Radial Sector (RS) still 2-branched. M2 vanished. M4 still present. Common stalk of CuA and 1A longer than in Sciopus, but still not reaching wing-margin. |

The way of life of the representatives of the dolichopodoid subtype is predatory, and with that they are in their [individual] development closely connected with water-basins and humid stations in which their larvae live. These flies usually possess powerful running legs (see Figure 8, above), which essentially are the principal organs of their locomotion. Flight plays, in a certain degree, a subordinated role.
2. Clythioid subtype.
The special diptera of the family Clythiidae [= Platypezidae] (Figure 5 and 5a) or fungus flies, usually considered (without serious basis) to be close to the hoverflies (Syrphidae) and the family Dorilaidae [= Pipunculidae], illustrate the peculiar clythioid subtype of muscoid wings, which [subtype] is characterized by a weak development of costalization (which [weak development] is expressed by the absence of shifts of the venation towards the anterior wing-margin), by the firm Subcostal vein, the absence of disconnections in the Costal vein, a long, as a rule, cubital cell, and a weak development of the alula. Anal lobe very large, often in the form of a rectangular outgrowth. Basiala wide, with moderately thickened handles of veins, while the lower element [= base of Cubitus and first Anal vein] is long, but notably approaching the handle of the second Anal vein. Phragma present as a rudiment in the form of a fold. The central field of the basiala is very large and wide. Cross-veins usually are present [in the wing-blade], but sometimes reduced. General size of the insect is small and varies between 1.5 and 5 mm. The wings are about equal in length to the body. Surface area of the wings is known only in two species of the genus Clythia and varies between 0.082 and 0.100 cm2. The load between 0.029 and 0.032 gr/cm2.
It is instructive to add some more images of flies and venation in the family Clythiidae :



Although it is relatively easy to find a venation that may formally be considered to represent a precursor of the groundplan of clythioid venation (as this groundplan is represented in Platypezina (Clythiidae)), where such a precursor might be for instance Spaniopsis (Rhagionidae) or Homalocnemis (Empididae), it is nevertheless not certain how precisely the clythioid venation has originated.


The clythioid subtype is of special interest because it is a clear link between the empidoid, syrphoid [later to be treated], and muscoid types. In fact, clythioid wings differ little from empidoid ones -- such as in the firm subcostal vein, small alula, absence of clearly expressed costalization in the form of weakening of [certain] veins, which all remain strong and colored, and finally, the pecularity of the branching of the anterior Media, sometimes forming a true apical cross-vein. All these listed features point to a smaller degree of mechanical specialization of the clythioid wings as compared to the microphoroid subtype and bears witness of their ancient origin from certain original forms of microphoroid wings.
3. Conopoid subtype.
The representatives of the family Conopidae possess wings of the special conopoid subtype (see Figures 6 and 7, above, and next two Figures).


Figure 13 : Wing-venation of Conopidae.
Left image : Conops flavipes L.
Right image : Sicus ferrugineus L.
(After HENNIG, 1954)
Absence of disconnections in the Costal vein, a long and firm Subcostal vein, a straight and simple anterior Medial branch, often ending up in the posterior Radial vein, presence of a rudimentary phragma in the basiala, variable length of the cubital cell, often long, almost reaching wing-margin, large alula, large cubito-anal handle in the basiala, weak development, almost absence of wing- and thoracic-scales, -- all this very well characterizes this subtype. In addition, characteristic of conopoid wings is their elongation : sometimes the length of the wing is more than three times greater than its width. Costalization as a whole is little expressed and consists in a softening [i.e. freeing of veins] of posterior wing-parts and in a certain thickening of the Costal and Radial veins. Overall size of the body is medium -- from 5 to 12 mm, rarely minute -- down to 3.5 mm (some species of the genera Occemyia and Dalmannia), more often large, sometimes up to 20 mm (some species of the genus Physocephala). Surface area of the wings and load are investigated only in one singe male of one species of the genus Conops and were equal to 0.294 cm2 and respectively 0.123 gr/cm2.
4. Anthomyioid subtype.
By a further strengthening of the processes of costalization of the wing is characterized the anthomyioid subtype.
To begin with, let us present some images of flies (Figure 14 and 15), the wings of which belong to the present subtype :

Figure 14 : Diptera-calyptrata. Representatives of the anthomyioid subtype of the lifting lightly-veined (muscoid) functional wing-type.
3 - Delia brassicae, Cabbage fly. 5.5-7.5 mm. Family Anthomyiidae.
8 - Fannia canicularis, Lesser House-fly. 5-7 mm. Family Anthmyiidae.
(After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977)

Figure 15 : Diptera-acalyptrata representing the anthomyioid subtype of the lifting lightly-veined (muscoid) functional wing-type.
Two images at the upper-left :
3 - Ceratitis capitata, Family Trypetidae [= Trypaneidae]. 4.5-6.5 mm.
4 - Rhagoletis cerasi, Family Trypetidae. 3.5-4 mm.
(After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977).
Remaining images, 3-15, after CHINERY in Elseviers insektengids voor West-Europa, 1983 :
3 - Melieria omissa Meig. Family Otitidae. About 10 mm.
4 - Urophora cardui L. Family Trypetidae [= Tephritidae = Trypaneidae]. About 7 mm.
5 - Calliopum aeneum Fall. Family Lauxaniidae. About 6 mm.
6 - Tetanocera elata Fabr. Family Sciomyzidae. About 7 mm.
7 - Platystoma seminationis L. Family Platystomidae. About 6 mm.
8 - Dryomyza flaveola Fabr. Family Dryomyzidae. About 11 mm.
9 - Coelopa frigida Fabr. Family Coelopidae. About 6 mm.
10 - Psila rosae Fabr. Family Psilidae. About 4.5 mm.
11 - Trepidaria petronella L. Family Micropezidae [= Tylidae = Calobatidae]. About 8 mm. (other species of this family belong to the tyloid subtype of muscoid wings).
12 - Chamaemyia aridella Fall. Family Chamaemyiidae. About 3.5 mm.
13 - Lonchaea chorea Fabr. Family Lonchaeidae. About 6 mm.
14 - Sepsis punctum Fabr. Family Sepsidae. About 7 mm.
15 - Piophila casei L. Family Piophilidae. About 5 mm.
The wings of all the species depicted here belong to the anthomyioid subtype of the lifting lightly-veined (muscoid) functional wing-type.
Next we depict examples of the wing-venation functionally belonging to the anthomyioid subtype :

Figure 16 : Venation of Trypeta tussilaginis ( Trypetidae), also belonging to the anthomyioid subtype of the muscoid functional wing-type. The posterior Medial vein is interpreted by the authors of the drawing as M3. In our interpretation, however, (as in that of ROHDENDORF and HENNIG), it is considered to be M4.
(After RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977)
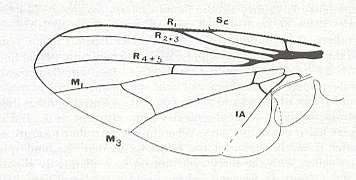
Figure 17 : Venation of Hylemyia strigosa ( Anthomyiidae), also belonging to the anthomyioid subtype of the muscoid functional wing-type. As regards the vein labelled M3, see subscript of previous Figure.
(After RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977)



Figure 18 : Lifting lightly-veined muscoid wings of representatives of the anthomyioid subtype.
From top to bottom :
Wings of this subtype are possessed by a very large number of fly species, belonging to the families Trypetidae [= Trypaneidae] ( Figure 18, second image), Sepsidae ( Figure 18, fourth image), the majority of Lauxaniidae, Sciomyzidae ( Figure 18, top image), Pyrgotidae, Cordiluridae, Anthomyiidae ( Figure 18, last but one image). To this subtype also belong the majority of representatives of Muscidae.
It is necessary to note that the majority of the listed families is taken by me [Rohdendorf] in a broad sense : Up to not long ago [reckoned from 1951] in the systematic literature on Diptera the formal [taxonimic] system of HENDEL was prevailing, in which the concept of the range of the category of f a m i l y of acalyptrate flies was taken artificially narrow.
Further do belong here certain Drosophilidae (i.e. a part of Ephydrinae, ( Figure 18, third image), some Larvaevoridae, for instance the genera Phytomyptera, Rhacodineura, and Phasiidae (genus Cinochira, see Figure 18, bottom image). Characteristic of anthomyioid wings is the straight or weakly curved (some Ulidiinae), but always lacking a vein-rudiment, anterior Medial vein. The intermedial cell (= discoidal cell) almost always large, bounded by the intermedial cross-vein. Cubital cell always closed (except in Ephydrinae, see Figure 18, third image), usually short, rarely a bit elongated and at its end stretched in the form of an acute angle (some Ulidiinae, Trypetidae, see Figure 18, second image, Pyrgotidae). The structure of the Costal vein is diverse : A series of groups possesses a whole, not interrupted, vein (Sciomyzidae, Figure 18, top image, Sepsinae Figure 18, fourth image, Lauxaniinae, many Trypetidae -- Otitinae, Platystomatinae, Ulidiinae), in other groups there is a single disconnection before the ending of the Subcostal vein (Pyrgotidae, Chamaemyiinae, Psilinae and some Trypetidae -- Richardiinae), and, finally, in many groups the Costal vein has two (Trypetinae, Figure 18, second image) or three (Anthomyiidae, Muscidae, Larvaevoridae) clear disconnections before the outflows in it of the humeral cross-vein, the Subcostal, and anterior Radial vein. The Subcostal vein is, as a rule, firm and clear, directly ending up in the Costa. Only sometimes it is reduced at its end and unifies with the wing-margin by a fold of the membrane (Trypetinae, Psilinae). The anterior Medial branch is straight, sometimes curved downwards (Pyrgotidae and Neottiophilinae) or directed upwards and converging toward the last Radial branch (Ulidiinae). The posterior basal and cubital cells well expressed, relatively large, the latter short and only sometimes stretched into a pointed form towards the wing-margin (some Trypetinae, Pterocallinae, Ulidiinae). Very rarely these cells are absent (some genera of Ephydrinae). Alula well developed, usually large, rarely of moderate size (some Opomyzinae). Wing and thoracic scales usually moderately developed, sometimes very large and projecting (Platystomatinae, Muscidae, Larvaevoridae, and Phasiidae), sometimes almost absent (Sepsidae, Sciomyzidae, Figure 18, top and fourth image). Body size, as a rule, minute -- from 3 to 7 mm, more rarely larger -- up to 15 mm (some Sciomyzidae). Only very rarely the body measures less than 3 mm (some Chamaemyiinae, Trypetinae, and others). Absolute size of the surface area of the wings and their load are known only for a few representatives of the families Anthomiidae, Sepsidae, Sciomyzidae, Trypetidae, and Lauxaniidae. In the only investigated specimen of a single species of the genus Sepsis the surface area was equal to 0.045 cm2, and the load 0.049 gr/cm2. Corresponingly, in three anthomyiids (genera Pegomyia and Paregle) 0.094-0.170 cm2 and 0.043-0.052 gr/cm2, in muscids (genera Fannia [which, however, also according to Rohdendorf -- see subscript of his Figure 42 (= our Figure 18), belongs to the family Anthomyiidae] and Pogonomyia) 0.115-0.251 cm2 and 0.056-0.073 gr/cm2, in the sciomyzid Trypetoptera 0.089 cm2 and 0.043 gr/cm2, in two trypetids Orellia 0.132-0.142 cm2 and 0.048-0.052 gr/cm2, and in the helomyzine Suillia 0.131-0.136 cm2 and 0.044-0.077 gr/cm2.
This subtype in fact includes the very main mass of forms of the lifting lightly-veined (muscoid) type, most widely represented in the recent fauna. It is a derivative of clythioid wings, and differs from this original subtype by processes of further perfection of aerodynamic qualities, first of all by the development of an elongation of the wings, development of features of costalization (in the form of an increase of veinless membrane at the wing's hind-margin, development of the anal lobe, scales, and [costalization features in the form of] a reduction of the hind-branch of the anterior Medial vein). Further specialization of the anthomyioid subtype has led to a whole series of other idiosyncratic subtypes [of muscoid wings] which are characterized chiefly by the development of processes of costalization or by a narrowing of the wing, about which we will speak later.
The biological features of the larvae of the representatives of this subtype is very diverse. Numerous are phytophagous species (for example Trypetinae, Psilinae, minute Anthomyiidae, and others). Also known are many forms of which the larvae live in various decaying plant-material (for instance Ulidiinae and Platystomatinae) or animal-material (for instance Sepsinae, Piophilinae, Muscinae). Finally there are rather many diptera among this subtype of which the larvae are predatory (for instance Chamaemyiinae) or parasitize in the body of insects (Phasiidae, Acridomyiidae, Pyrgotidae, and others) or in molluscs (some Sciomyzidae).
Flight in insects with anthomyioid wings is rather diverse, and as a whole is characterized by high qualities. However, one can by far not maintain that in all representatives of the subtype flight is uniformly perfected and plays a similarly important role in life-activity. Many species of phytophagous and vegetable saprophags [consuming vegetable debris] (for instance Trypetidae in the broad sence and species of Sciomyzidae) fly little and spend much of their time on the plants. Some parasitic and predatory forms, and also saprophags (for instance many Anthomyiidae, Muscidae, Larvaevoridae), consuming animal matter, fly significantly better and more frequently. Finally, some representatives of the subtype (for instance Sepsinae, many Trypetidae, and others), while crawling produce special peculiar slow movements (some sort of vibration and fanning with the streched-out wings) of which the physiological meaning is unclear.
The diversity of living-conditions of the representatives of the subtype undoubtedly is the cause of the rather wide range of variability of anthomyioid wings, among which one can see, in addition to moderately elongate wings of, for instance, Trypetidae (see Figure 18, second image and Figure 16), the [still] longer wings of the Pyrgotidae or Sepsidae (Figure 18, fourth image). On the contrary, some other groups illustrate a shortening and widening of wings (certain Lauxaniidae). Also variable are the degree of costalization and the development of disconnections in the Costal vein. The whole picture of the variability of the anthomyioid wings clearly points to the paths of formation of other subtypes, their derivatives.
Before we move on (following ROHDENDORF's text) to the next functional subtype of muscoid wings (the muscoid-proper subtype), we insert some considerations that may guide us how to theoretically digest ROHDENDORF's exposition of functional subtypes of wings in predominantly acalyptrate flies :
Wing-structure and living-conditions
We just heard from ROHDENDORF that the "diversity of living-conditions of the representatives of the subtype undoubtedly is the cause of the rather wide range of variability of anthomyioid wings". At first sight this may sound more or less contradictory because the apparent great uniformity [instead of diversity] of wing-structure in the representatives of the anthomyioid subtype would seem to contrast with the extraordinary diversity [multiformity] of living-conditions of these flies at least insofar as these conditions are related to their individual development [egg-larva-adult]. The developmental living-conditions of acalyptrate flies -- many of them belonging to the anthomyioid subtype (and many others to other subtypes) -- range from compost-feeding (which is the original feeding habit of the larvae in this group), via phytophagy (including parasitism in plants) and predation, to parasitism in animals (insects and molluscs), while, on the other hand, the wing-venation of all these flies (see, for instance, Figure 15) conforms to a single common master-plan : Radial Sector 2-branched, Media 2-branched, the anterior branch of which is straight [i.e. not markedly curved], a large discoidal cell, a very short cubital cell, a developed alula, and a low degree of costalization. So the "rather wide range of variability of anthomyioid wings" of which ROHDENDORF speaks must refer to the more subtle details of the wing (and its basiala) which indeed are described by him. The differences between these details must determine corresponding (small) differences in flight-regime, itself in turn evolutionarily determined by the mentioned differences of living-conditions (i.e. differences of individual development and all that is directly connected with it). Adult flies must seek and find appropriate substrates for their larvae, and this largely determines (among other things) the flight-regime. So in this particular form we can agree with ROHDENDORF that the diversity of anthomyioid wings is evolutionarily determined (in the form of the appearance of adaptations) by the diversity of living-conditions of these flies and their larvae.
Biocoenoses are more or less independent organismic communities (micro-organisms, plants, animals) in the Explicate Order. Each one of them, together with the inorganic conditions in which such a biocoenosis is embedded, forms a pattern of potential and actual existential conditions of a number of organismic species. So the existential condition of a given organismic species is its biocoenosis (to which it belongs as a member) plus the biocoenosis' inorganic existential conditions (climatic factors, presence and quality of water and minerals, relief of the terrain, and so on). Central in our exposition is not the biocoenosis as such, but a given organismic species in it. The structure, physiology and behavior of the individuals of such a species together constitute the materialized existential strategy of that species. This strategy (adaptations and the like) contains internalized biotic and abiotic environmental factors, where "internalized" means that these factors are morphologically and physiologically expressed or reflected in the organism (for example, a hard and compact feeding substrate is reflected in powerful toothed jaws). So such a strategy includes in fact these environmental conditions. And the true (i.e. external) environmental conditions plus their internalizations (that constitute the strategy) together form the ecological niche of this organic species in its biocoenosis.
For understanding organic evolution (in geologic time) the biocoenoses and their abiotic conditions are of considerable importance : i.e. the change in geologic time of these biocoenoses plus their abiotic conditions (this evolution not considered by Schwerdtfeger) should be followed (on the basis of paleophytologic, paleozoologic, paleogeographic, and paleoclimatologic investigations), whereby in our approach of things the organismic species as a materialized existential strategy is central (see also, for instance, ROHDENDORF and RASNITSIN, in "The historical development of the class of insects" [in Russian], 1980).
We have theorized that the actual structural development and creation of new species, i.e. of new existential strategies, take place polyphyletically in the Implicate Order, whereas the consecutive [order of] appearance of them, as organismic species, into the Explicate Order depends on the existing pattern there of true existential conditions, that is of appropriate biocoenoses in their inorganic conditions. The evolution of acalyptrate flies is determined by transitions from compost-feeding to substrates with higher nutritive content (see ABOVE). And, as has been said, these transitions determine corresponding transitions from one particular flight-regime to another, expressed by the appearance of subtle differences of wing-structure (and also determine other structures as well, such as a specific type of legs and sensory organs of adult flies, and the physiological and morphological structures of their larvae). These transitions in wing-structures of acalyptrate flies (and their relations to those of calyptrate flies [where breeding in animal matter is predominant] ) are later described in more detail by Rohdendorf, to which we will return after having completed Rohdendorf 's description of all the 14 subtypes of muscoid wings.
This brings us to the following special ecological considerations.
Biocoenoses and existential conditions in the Explicate Order
The biocoenosis plus all its inorganic conditions forms, as has been said, a pattern of potential and actual existential conditions for a multitude (not as multitude) of organismic species, that is, it forms a multitude of ecological niches.
Absolutely necessary for any given organismic species to be able to exist (in the Explicate Order) is the cycle of convertents in the biocoenosis : producers (producents) of organic material, consuments of [that] organic material, and reducers (reducents) of organic material eventually all the way down to inorganic material again. This cycle is, as has been said, a necessary general existential condition for every member (plant or animal) of a biocoenosis. One or more links in this cycle may be absent without disrupting things, provided the cycle is still truly circular, i.e. beginning with the presence of inorganic materials it ends up with these materials again so that they do not become exhausted.
When all possible links are present we then have :
Inorganic materials plus solar radiation, are transformed by autotrophic organisms such as green plants (producents) into living plant-matter, which either by itself decays into dead organic matter after the plant has died (because plant-matter as such, i.e. outside a living organism, is unstable), or is, by the action of phytophagous and zoophagous organisms (consuments), transformed also into dead organic matter (carrion and excrements). This dead organic matter is then further processed into more simple dead organic matter (processed carrion and processed excrements) by necrophagous, saprophagous, and coprophagous organisms (also consuments), which processed organic matter is then, finally, processed by bacteria and fungi (reducents) and transformed into inorganic matter again, which itself can again be used by producents.
We see that the whole gamut of living conditions of (the larvae of) acalytrate flies fits into this pattern, between the green plants and the bacteria and fungi. See for more details SCHWERDTFEGER, Synökologie, 1975, p.268/269, and his Figures 83 and 84.
All inorganic structures and processes belong to the Explicate Order. Perhaps crystallization is connected with the Implicate Order. But also the formation of crystals belongs to spontaneous processes thermodynamically leading to an energy-minimum. So crystals and crystallization are not strategies but passive formations. An organism, on the other hand, does not seek an energy-minimum, but a c t i v e l y keeps itself far from it. Only after death there is a spontaneous course to an energy-minimum (thermodynamic equilibrium). So an organism is a representative of a strategy, the strategy of the species to which it belongs. Feeding by an organism serves to keep it far from thermodynamic equilibrium, and holding it at a biologic equilibrium. And it is the appropriate biocoenosis in which this is possible.
To the domain of the Explicate Order do belong the creation of new existential conditions of organismic species, and the destruction of them, reflecting the c h a n g e of biocoenoses plus their abiotic conditions in the course of shorter or longer periods of time (one of the main dimensions of the Explicate Order). Extinction of organismic species, and migration of populations (of species) also belong to the domain of the Explicate Order. Insertion of new species in existential conditions that have become vacant or have been newly created belongs to, or at least involves, the Implicate Order.
In Rohdendorf 's view the transition from one functional subtype of muscoid wings into another (and also from one chief functional type into another chief type) is superimposed upon (and so does not contradict) the overall phylogenetic tree of those flies.
Such a tree (not necessarily strictly built on HENNIG's principles of phylogenetic systematics) represents the (assumed) genealogical relationships between existing and having existed organismic species, not between (functional) types or subtypes. The affiliation (derivation) of these types and subtypes (as is done in Rohdendorf) is, as has been said, superimposed upon the genealogic tree, and does not generally involve theoretical contradictions. For example (anticipating the exposition of two more subtypes of muscoid wings) :
A species of the family Muscidae, a species happening to belong to the muscoid-proper functional subtype of muscoid wings (not as a result of it systematically belonging to the family Muscidae) is evolutionarily transformed into a species of the family Larvaevoridae, and now (not because of it belonging to the family Larvaevoridae) belonging to the tachinoid functional subtype of muscoid wings. Then the same, or another, species of Larvaevoridae (and also belonging to the tachinoid wing-subtype) is transformed into another species of Larvaevoridae, but now functionally belonging to the muscoid-proper functional subtype of muscoid wings. So, in summarizing, we have here a member of the family Muscidae transforming into a member of the family Larvaevoridae, and then further being transformed into another member of the family Larvaevoridae. This is the phylogenetic picture, which does not contradict the two-way derivation between the muscoid-proper wing-subtype and the tachinoid wing-subtype (muscoid ==> tachinoid ==> muscoid).
In our theory, on the other hand, all organismic species develop polyphyletically (and noëtically) in the Implicate Order, while their order of appearance in the Explicate Order depends on the presence of appropriate existential conditions in that Order, and consequently [depends on] the evolution and origin -- in the Explicate Order -- of biocoenoses in which the mentioned cycle of convertents is closed.
Figure above : Diagram of morphoklines in a general way depicting the noëtic evolution of the strategies of acalyptrates belonging to the anthomyioid subtype of muscoid wings.
The mentioned noëtic development in the Implicate Order of acalyptrate flies is of the semi-independent type : Noëtic development of strategies along separate lines running from one noëtic counterpart of existential conditions to another, and so is the development of the acalyptrate species belonging to the anthomyioid subtype of muscoid wings :
Generally, there has taken place, in the Implicate Order, a transition of a given strategy from one noëtic counterpart of possible existential conditions to another, resulting in a new strategy (without exhaustion of the original strategy). These noëtically constructed and transformed strategies will be projected into the Explicate Order when the corresponding material existential conditions are actually present in that Order, i.e. when the appropriate vacancies in the relevant biocoenosis (or biocoenoses) are actually present.
In the present case of acalyptrate flies with wings belonging to the anthomyioid subtype of the muscoid type the mentioned ecological vacancies involve several possible breeding media such as (1) compost, (2) more fresh [than the latter] material of decaying plants (for instance parts of plants a short while ago already attacked by other insects), (3) fruits (decaying or fresh), (4) parenchym of leaves (obtained by mining in them), (5) galls on plants, and (6) living animals (obtained by predation or parasitism). It is supposed that generally there is an evolutionary tendency toward media (i.e. feeding-substrates) that contain higher concentrations of nutritive elements. But also reverse courses cannot excluded to have taken place (involving, of course, other species). And because (a) the way of life, and especially the larval nutritive substrates of many species of the present subtype (and all the next ones) are badly or not at all known, and (b) the almost uniform wing-venation in this group does not allow to set up reliable derivational relations (derivational lines), we will, in the evolutionary assessment of this subtype, and also in that of the remaining subtypes, give up to set up specified derivational lines and morphoklines and limit ourselves to give merely a general non-specified diagram of morphoklines. And because we assume that all organismic species have developed polyphyletically we can, in the case of Diptera with muscoid wings, happily arrange them in the diagrams of morphoklines according to Rohdendorf s subtypes. Within the acalyptrates nothing is known about the order of appearance of the species in the course of time, -- also a reason not to specify these diagrams. Therefore here the symbols A, B, C, D, etc. (no relation with the same symbols in any other such diagram) just refer to undetermined species of those acalyptrates that belong to the anthomyioid subtype of muscoid wings, and also the ecological zones in the diagrams are not specified. They just generally refer to the just mentioned breeding media without having them specified.
Further elaborating on all this, we may conclude that with respect to all the following subtypes of muscoid wings it is even not necessary to present the mentioned mere unspecified derivational lines and morphoklines at all. And that's what we do.
(Continuing with Rohdendorf 's text)
5. Muscoid-proper subtype.
As the special muscoid-proper subtype (of the muscoid type) do represent themselves the wings of certain species of the families Muscidae (subfamily Muscinae, see first and third images of the next Figure), many Larvaevoridae (namely a large number of Calliphorinae, for instance of the genus Booponus [and Calliphora (see Figure 21), many Dexiinae (see Figure 20), Larvaevorinae, for instance of the genera Thyella, Ernestia (last image of next Figure), Exorista, Actia and others) and, finally, Phasiidae (second image of next Figure).



Figure 19 : Lifting lightly-veined wings of representatives of the muscoid-proper subtype of muscoid wings.
Top image - Wing of Graphomyia maculata Scop., Muscidae (Calyptratae). Length 9.5 mm.
Second image - Wing of Phasia crassipennis Fabr., Phasiidae (Calyptratae). Male. Length about 11 mm.
Third image - Wing of Orthellia sp. Muscidae (Calyptratae). Length 8 mm.
Bottom image - Wing of Ernestia consobrina Meig., Larvaevoridae (Calyptratae). Length 10 mm.
(After ROHDENDORF, 1951)

Figure 20 : Lifting lightly-veined wing of a representative of the muscoid-proper subtype of muscoid wings
Myiostoma sp. Larvaevoridae, Dexiinae (Calyptratae). Apical half of right wing of female. Length of wing 11.8 mm.
Well visible is the rudiment of the Medial branch [i.e. the rudiment is of the second branch of the anterior Medial vein].
(After ROHDENDORF, 1951)

Figure 21 : Wing-venation of Calliphora erythrocephala. Calliphoridae (or, if one wants, Larvaevoridae, Calliphorinae) (Calyptratae). It also belongs to the muscoid-proper subtype of muscoid wings. The posterior Medial vein is interpreted by the authors of the drawing as M3. In our interpretation, however, (as in that of ROHDENDORF and HENNIG), it is considered to be M4.
(After RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977)
Very characteristic of this subtype is the presence of a strong curve of the anterior Medial branch, coming close to, or even unifies with the last Radial branch, together with a moderate development of vein-free areas of the membrane along the wing's hind-margin. The costal vein always has disconnections or at least thinnings and transparent points in three parts -- before the humeral cross-vein, directly after it, and before the inflow of the Subcostal vein. The edge of the wing is covered with firm but lying bristles, of which sometimes one, rarely two, lying close to the end of the Subcostal vein, are projecting and lengthened. The anterior Medial branch is always curved at its distal section, forming the so-called apical cross-vein. This vein borders a very characteristic cell of the wing (carrying the name of fifth radial), which is very large and wide, a l w a y s w i d e r than the free membrane between this cell and the posterior wing-margin. Costalization is relatively weakly expressed, showing itself by the widening of the anal lobe and strengthening of the costal vein. Virtually no shifts of veins have taken place. Alula, wing- and thoracic scales very large. Subcostal vein always firm and complete. Cubital cell very short. Intermedial cell [= discoidal cell] large. Absolute size usually medium or large, about 4 to 10 mm, rarely minute (about 3 mm -- Alophora (P.) pusilla Meig.). Magnitudes of surface area and load of the wings are investigated only in some muscids, calliphorines, and one phasiid. Thus, in Muscidae (genera Pyrellia, Orthellia, Morellia, Muscina, and Graphomyia) the surface area was equal to 0.121-0.340 cm2, the load equal to 0.085-0.184 gr/cm2. In the phasiid Alophora obesa F. -- 0.113 cm2 and 0.075 gr/cm2. In calliphorines (genera Cynomyia, Calliphora, and Pollenia) the surface area was equal to 0.271-0.751 cm2, the load to 0.058-0.139 gr/cm2. Finally, in certain Tachininae (genera Ernestia, Thyella, Exorista) the surface area was equal to 0.201-0.0624 cm2, the load to 0.075-0.147 gr/cm2.

Figure 22 : Representatives of the muscoid-proper subtype of muscoid wings.
1 - Larvaevora fera L. about 15 mm. Tachinidae [or, if one wishes, Larvaevoridae, Tachininae] (Calyptratae).
2 - Gymnochaeta viridis Fall. about 12 mm. Tachinidae. (Calyptratae).
3 - Salmacia divisa Meig. about 13 mm. Tachinidae. (Calyptratae).
4 - Alophora hemiptera Fabr. Tachinidae. (Calyptratae). 4a - male, about 15 mm. 4b - female, about 12 mm.
5 - Lucilia caesar L.. about 11 mm. Calliphoridae [or, if one wishes, Larvaevoridae, Calliphorinae]. (Calyptratae).
6 - Calliphora vomitoria L. about 12 mm. Calliphoridae. (Calyptratae).
7 - Pollenia rudis Fabr. about 7 mm. Calliphoridae. (Calyptratae).
8 - Sarcophaga carnaria L. about 15 mm. Sarcophagidae [or, if one wishes, Larvaevoridae, Sarcophaginae] (Calyptratae).
10 - Mesembrina meridiana L. about 12 mm. Muscidae. (Calyptratae).
11 - Stomoxys calcitrans L. about 11 mm. Muscidae. (Calyptratae).
12 - Dasyphora cyanella Meig. about 11 mm. Muscidae. (Calyptratae).
13 - Musca domestica L. House-fly, about 9 mm. Muscidae. (Calyptratae).
(After CHINERY, in Elseviers insektengids voor West-Europa, 1983)
Looking at the variability of the muscoid-proper wings, one may distinguish diverse changes in this subtype first of all expressing the different origin of its representatives. The morphological expression of these relationships consists in the different forms of the bend of the Medial branch : (1) arched, for instance in some Phasiidae, Calliphorinae (genus Booponus), or Muscidae (genera Muscina, Stomoxys, Pyrellia), (2) in the form of an obtuse angle, for instance in the genera Musca, Orthellia, Lucilia, Phasia, and many others, and, finally, (3) right-angled, as in some Larvaevorinae and Calliphorinae. This feature is only partly connected with the development of more or less costalization, [i.e.] with the widening of vein-free fields of the membrane of the posterior marginal part of the wing (by which this subtype is distinguished from tachinoid wings [i.e. wings of the tachinoid subtype, later to be considered] ) [ It was said : "only partly connected with (...) costalization, [i.e.] with the widening of vein-free fields (...)" -- because here, in the muscoid-proper subtype, it [the M-bend] often is merely a matter of atavism, i.e. a retention of an ancient feature, meaning that it is the source or origin, and not some costalization-process, that is responsible for the M-bend].

The tachinoid subtype will be considered later on.
6. Tyloid subtype.
The peculiar wings of the representatives of the family Tylidae [= Micropezidae] (second image of next Figure) and some Lauxaniidae (the genera Anthomyza, Opomyza, Geomyza [first image of next Figure], and relatives) form the characteristic tyloid subtype of the muscoid type.


Figure 23 : Lifting lightly-veined wings of representatives of the tyloid [first two images], agromyzoid [3rd anf 4th images], and the chloropoid subtypes [last two images]. From top to bottom :
A - Wing of Geomyza angustipennis Zett., Lauxaniidae. Length about 2 mm. (After RUBZAMEN in CZERNY). ( Tyloid subtype).
B - Wing of Tylos corrigiolatus L., Tylidae (superfamily Psilidea). Length 4 mm. ( Tyloid subtype). (After RODHENDORF, 1951)
C - Wing of Phytomyza kamtschatkensis Hend., Agromyzinae. Length about 1 mm. ( Agromyzoid subtype). (After HENDEL)
D - Wing of Agromyza frontella Rond., Agromyzinae. Length about 2 mm. ( Agromyzoid subtype). (After HENDEL).
E - Wing of Chlorops sp., Chloropidae. Length 2.5 mm. ( Chloropoid subtype). (After ROHDENDORF, 1951).
F - Venation of Chlorops taeniopus, Chloropidae ( Chloropoid subtype). (After RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977).
Adding some pictures of flies at least related to those of the tyloid subtype :

Figure 24 : Opomyza germinationis L. Opomyzinae ( Lauxaniidae),. about 4 mm. ( It might well be that the wings of this fly belong to the anthomyioid instead of to the tyloid subtype, because they are not markedly elongate (compare with the first two images of Figure 23 (true tyloid subtype) and with Figure 18, last image (true anthomyioid subtype).
(After CHINERY, in Elseviers insektengids voor West-Europa, 1983)


Figure 26 : Anthomyza gracilis Fall. Anthomyzinae ( Lauxaniidae). about 3 mm. ( Also this fly might well be a representative of the anthomyioid instead of the tyloid subtype). (After CHINERY, in Elseviers insektengids voor West-Europa, 1983)
Tyloid wings are characterized by a very peculiar shape, reminding us of the wings of the corynetoid subtype of empidoid wings [see, for instance Dysaletria atriceps (Empididae) or Coryneta stigma (Empididae).] Length of the wings from 3 to 4 times longer than their width. Fore- and hind-margin almost in the same degree convex. Anal lobe weakly developed (genera Anthomyza (Figure 26) and Opomyza ( Figure 24 ), sometimes completely absent (genus Geomyza, A in Figure 23 ). Also very small is the alula, and the [wing- and thoracic-] scales are almost completely absent. Base of wing narrowed. Venation not costalized, the veins lie parallel in the wing, whereby the simple Medial branch sometimes approaches with its distal section the posterior Radial branch (some Tylidae). Intermedial (= discoidal) cell usually short and only in Tylidae long. Costal vein without disconnections in representatives of the Tylidae and with clearly expressed disconnections in other forms. Cubital and posterior basal cells large, always standing out clearly. Basiala with a very wide central lower field. Absolute size of these insects small, from 2 to 6 mm, rarely up to 10 mm or even larger (tropical Tylidae). Wings usually short, sometimes almost reduced (genus Geomyza). Magnitudes of surface-area and load have not been studied in these flies.
7. Agromyzoid subtype.
Mining-flies, representatives of the family Agromyzidae, and some other closely related groups (Milichiidae and Odiniidae) possess characteristic wings, together making up the special agromyzoid subtype. See for wings and venation Figure 23, 3rd and 4th image, and for the flies the next three Figures.


For easy reference we here reproduce the two agromyzoid wings of Figure 23 :

Characteristic of this subtype is the development of costalization, which expresses itself by the shift into the direction of the wing-base and reduction of the cross-veins together with the weakening of the longitudinal veins of the posterior half of the wing and a certain thickening of the anterior veins. The Costal vein is strong, not reaching the wing-apex, and carries a sharply expressed disconnection at the place where the Subcostal vein merges with the Costal (the Subcostal vein is in fact a mere weak fold, its reduced rudiment). The two branches of the Radial Sector firm and straight, almost always long, only weakly diverging. Medial veins always straight, long, and not shifted anteriorly. Sometimes they are markedly thinner than the Radial veins (genus Phytomyza). Intermedial cell, as a rule, short, far from reaching the wing's midpoint. Seldom this cell is comparatively large (some species of the genera Agromyza ( Figure 29D ), Ophiomyia, Milichia, and Odinia). Sometimes the intermedial cell is absent as a result of the reduction of the intermedial cross-vein (genus Phytomyza, Figure 29C and certain others). Anal-lobe and alula [the Russian text reads "wing-scale"] well developed. The shape of the wings is rather broad : length 2.25-2.75 times larger than the wing's width. The vein-free parts of the wing are very wide. As a result of the reduction of the intermedial cross-vein and the shift of the radio-medial cross-vein toward the wing-base, the whole wing-blade becomes very flexible [as a result of the absence of cross-veins in its chief part], strengthened only by the strong Medial and Radial veins. The absolute size of these insects is very small, often hardly surpassing 1 mm and never larger than 3-4 mm. The size of the wings equal or smaller than the body. Magnitude of surface-area and load of the wings were not investigated.
8. Chloropoid subtype.
The large family of grass-flies, Chloropidae, illustrates the peculiar chloropoid subtype. See next three Figures (two of them already given earlier).

Figure 30 : Wing of Chlorops sp., Chloropidae. Length 2.5 mm. chloropoid subtype of muscoid wings.
(After ROHDENDORF, 1951)

Figure 31 : Venation of Chlorops taeniopus, Chloropidae ( Chloropoid subtype). According to our interpretation the vein labelled M3 is in fact M4. (After RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977).

Figure 31a : Oscinella frit, Chloropidae. about 3 mm long. ( Chloropoid subtype).
(In RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977).
Most characteristic of the wings in this subtype is the development of strong costalization, realized by way of thickening and shifts toward the anterior wing-margin of the Radial veins [especially of R1 and R2+3]. The Costal vein is very strong, far from reaching the wing-apex, and carries one sharp disconnection before the place where R1 ends up in the Costa. Subcosta almost completely reduced, leaving a mere trace. The three Radial veins are strong and always in some degree arched and shifted toward the anterior wing-margin. Medial veins rarely strong, more often significantly weakened. The intermedial cell is usually short, seldom reaching the wing's midpoint. The posterial basal and cubital cells are absent as a result of the reduction of the basal cross-vein (= basal part of the posterior Medial branch) and [of the reduction of] the end section of the Cubital [and of the reduction of] the first Anal vein. Anal lobe and alula almost always well developed, as well as the wing-scale. The posterior vein-free area of the wing-membrane very wide. The wing is not particularly short, towards its end usually with a narrowed apex. Absolute size of the grass-flies (Chloropidae) small : 1-4 mm, seldom up to 7 mm (only representatives of the genus Lipara). The wings are short, equal or shorter than the body. Forms with significantly shortened wings are known. Surface area and load of the wings were not investigated. Chloropoid wings are in many respects similar to those in the agromyzoid subtype, differing from them only by their higher degree of costalization. Also in their life-history the grass-flies are in many respects similar to the mining-flies (Agromyzidae), largely being plant-feeding forms. Their larvae live in grasses and in some other plants.
9. Drosophiloid subtype.
The diverse representatives of the extensive family Drosophilidae -- the subfamilies Drosophilinae (next Figure (32), top image), Diastatinae, Camillinae, Periscelidinae (next Figure (32), middle image), Tethininae, Aulacogastrinae, partly Ephydrinae, and Canaceinae (next Figure (32) bottom image), and of some Lauxaniidae (Clusiinae, Chiromyiinae) -- possess wings of the special drosophiloid subtype.

Figure 32 : Wings of the drosophiloid subtype.
Top image : Wing of Drosophila sp., Drosophilinae. Length 4.5 mm. (After RODHENDORF, 1951).
Central image : Wing of Periscelis annulipes Lw., Periscelidinae. Length 3.75 mm. (After DUDA).
Bottom image : Dinomyia ranula Lw., Canaceinae. Length about 1.75 mm. (After BECKER).
(From ROHDENDORF, 1951)
These wings may be compared with those of two other subtypes :

Figure 33 : Wings of the asteioid subtype.
Top image : Wing of Astiosoma rufifrons Duda, Asteiinae. Length 2.25 mm. (After DUDA).
Bottom image : Wing of Asteia concinna Meig., Asteiinae. Length about 2.8 mm. (After DUDA).
(From ROHDENDORF, 1951)
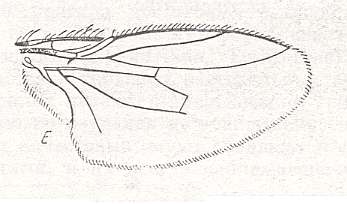
Figure 34 : Wing of the Sphaeroceratoid subtype.
Wing of Limosina sp., Sphaeroceratinae. Length 3 mm.
(After ROHDENDORF, 1951)
The most characteristic features of the drosophiloid subtype are : processes of reduction of the posterior basal and cubital cell, shortening of the intermedial cell, and a general shortening of the wing as a whole. The listed features, however, are far from having developed uniformly in all representatives of the subtype. Costal vein usually long, reaching wing-apex and ending at the end of the last Radial or even at the end of the anterior Medial vein. Usually there are from one to three disconnections in the Costal vein, seldom there are none (Clusiinae). The anterior Radial vein is stronger than the other [radial veins]. The other Radial veins and the anterior Medial vein are straight, thin, almost of the same thickness. The intermedial cell is very seldom longer than half the length of the wing-blade, often shorter. The posterior basal cell [= the cell just above the cubital cell] is usually coalesced with the intermedial cell (the cross-vein between them being reduced), sometimes it is present (Lauxaniidae, Periscelidinae, Canaceinae, Tethininae). Cubital cell usually present, more rarely reduced (Camillinae, Periscelidinae, Ephydrinae). Costalization of the wing is not particularly sharply expressed, but notably stronger than in the original anthomyioid subtype. Surface area and load of the wing is investigated only in one ephydrine [Ephydrinae] (genus Dichaeta -- surface area equal to 0.100 cm2 and load 0.068 gr/cm2) and in a drosophiline (genus Drosophila - 0.097 cm2 and 0.056 gr/cm2). Absolute size of the wings is variable, sometimes larger than the body (for instance in Drosophilinae), sometimes shorter (many Ephydrinae). Body size small, usually from 2 to 4 mm, sometimes minute, not above 0.75 mm (some Ephydrinae), more rarely reaching 5 mm.

The formation of the drosophiloid subtype was, apparently, determined by processes of decrease of body-size, together with the development of moderate costalization. These processes did not stand out as especially intensive, and in the evolution of the representatives of the subtype the determining changes were undoubtedly the perfection of other organ-systems. The flight abilities were improved only insignificantly and played a wholly subordinated role in their life-activities.
10. Asteioid subtype.
A special small group of flies, belonging to the family Drosophilidae, namely the minute flies of the subfamily Asteiinae, possesses peculiar muscoid wings, together constituting the asteioid subtype (see Figure 33 ), which is characterized by a shift and shortening of the middle Radial vein [= anterior branch of the Radial Sector] and by the approach to each other [i.e. running parallel] of two long veins (posterior Radial and anterior Medial), running through the mid-part of the wing. The Costal vein in its basal part, up to the place of inflow of the first Radial vein, very thin, but without clear disconnections. It reaches the wing-apex. Distal part of Subcosta reduced. First and second Radial veins short and running very close to each other (only in the genera Leiomyza and Uranucha the second Radial vein is long -- these genera represent a transition from the original drosophiloid subtype). The posterior Radial and anterior Medial vein very long, almost reaching the very wing-apex. Both are weakly curved, moreover the Medial notably approaches the Radial vein (= converges). The cross-vein [i.e. r-m] strongly shifted towards the wing-base. The intermedial cell is significantly shorter than half the wing-blade, often open, as a result of the reduction of the intermedial cross-vein (species of the genus Asteia) (see Figure 33, bottom image ). The posterior basal cell coalesced with the intermedial cell. The cubital cell is completely absent. Anal lobe of variable shape, often large -- genera Phlebosotera, Astiosoma (see Figure 33, top image ), Leiomyza and Uranucha, sometimes small and very smooth (genus Asteia). Alula weakly developed, somtimes completely missing -- genus Asteia (see Figure 33, bottom image ). Wing- and thoracic-scales rudimentary. Absolute size of the insect small -- from 1.25 to 3 mm. The relative size of the wings is large, the latter are longer than the body. The magnitudes of the surface area of the wings and their load are not investigated.
Asteioid wings are peculiar specialized forms of the drosophiloid subtype. Most peculiar in this subtype [the asteioid subtype] is the shape of the elongate wing, of which the blade is essentially strengthened only by two long converging veins. Interesting is the connection in this subtype of the working-out of special narrow wings devoid of outgrowths at the posterior margin, with a shift of the Radial branch [the second, that is] and the radio-medial cross-vein [r-m] toward the base of the wing, that is, features of true costalization that usually lead to the development of an overall transformation of the whole skeleton of the wing. However, asteioid wings have developed wholly into a special direction, not having changed into sharply costalized organs as we see them for instance in the agromyzoid or chloropoid subtypes. Phenomena of regressive development of the flight-function in this subtype undoubtedly did not take place. Of this we are convinced first of all by the increase of size of the wings. The life-history of Asteiinae is very little known : metamorphosis and nutritive substrate of the larvae are up until now [1951] unknown. We may only assume the larvae being phytophagous, [this supposition] based on the conditions in which the winged insects live. These flies (more precisely, species of the genus Asteia) can always be found in mixed meadows, rich in grasses, all over the taiga [= northern pine forests] and broad-leaved zones. The larvae probably live in the tissues of living plants (species of the genus Leiomyza in the fruit-bodies of higher fungi).
Flight of the Asteiinae is totally unstudied. Evaluating the nature of the morphological changes having led to the formation of the asteioid subtype out of drosophiloid wings, one must reckon these forms of the muscoid type as to be a specialized direction of perfection of the flight-function in minute flies, being determined by increase of surface area of the wings without a markedly increase of wing-beat frequency, consequently without improvement of governability during low speeds of flight. Such a kind of evolutionary direction of the flight-function is very unusual in minute insects. Therefore the given suppositions about the properties of flight in asteiines are in a certain degree preliminary and are in need of an exact verification.
11. Sphaeroceratoid subtype.
The characteristic diptera Sphaeroceratinae [= apparently Sphaerocerinae = apparently Borborinae] of the family Drosophilidae (see Figure 34 ) possess wings together making up the special sphaeroceratoid subtype, which most sharply differs from all other muscoid wings by the thickening of veins at the anterior margin and the central part of the wing (including also the Medial system!), together with the development of costalization phenomena. The Costal vein is very strong, usually not reaching the wing-apex, more rarely going up to the apex and the place of outflow in the wing-margin of the anterior Medial branch (genera Sphaerocera and Crumomyia). The Costal vein always has from one (for instance the genus Crumomyia) to three disconnections (for instance the genus Limosina). Subcosta largely reduced, lying against the Radial vein. The anterior Radial vein [R1, the Radius proper] very strong and simple, arched. The middle Radial branch [i.e. the anterior branch of the Radial Sector], as a rule, strong, fairly long, at its end curved. Seldom this vein weakened (some species of the genus Sphaerocera). The posterior Radial vein almost straight, very long. Always well expressed is the closed intermedial cell. The anterior Medial vein sometimes strong up to its end, sometimes weakened or even completely reduced from the intermedial cross-vein onwards. About the same takes place also with the posterior Medial vein. Cubital cell usually open, more rarely closed by the distal section of the Cubital vein (genera Sphaerocera and Crumomyia). Anal vein almost always clearly present. The posterior margin of the wing is devoid of veins, broad. Anal lobe and alula very large, sharply projecting. The anterior margin of the wing is provided with strong projecting bristles. Wing-scale present, but small. Absolute size of the body from 0.5 to 5.5 mm, usually from 1.5 to 3 mm. Relative size of the wings small : they are equal or a little shorter than the body. Magnitude of surface area and load is investigated only in two representatives of the genera Crumomyia (0.093 cm2 and 0.041 gr/cm2) and Limosina (0.050 cm2 and 0.042 gr/cm2).
Sphaeroceratoid wings undoubtedly are narrowly specialized forms of the muscoid type, having reached a high degree of costalization, together with the development of disconnections in the Costal vein. The pecularity of the sybtype consists in the retention of a strong venation in the center of the wing-blade in the form of a large intermedial cell (still see Figure 34 ), formed by strong Medial veins (up until now we saw examples of strongly costalized muscoid wings : but in them the processes of the transformation of the skeleton always involved the intermedial cell which either was shortened or became open as a result of the disappearance of the cross-vein). Sphaeroceratoid wings may be comparable only partly to stratiomyioids in which closed cells in the wing-blade were preserved despite intense development of processes of costalization [See for the stratiomyioid Hermione locuples HERE ]. This characteristic feature of sphaeroceratoid wings forces us to highly evaluate their mechanic properties, indicating a high degree of firmness of the wing-blade.
The biology of these flies is still little known : Their larvae develop in various decaying materials, of animal as well as of plant origin. The winged insects inhabit shady and moist stations rich in vegetation. However, many dung and carrion forms live in diverse conditions, often in rather dry and sun-lit environments. Moreover, inhabitants of hidden edaphic [soil-] spaces and various natural cavities and hollows in the soil are known. It is necessary to point to the presence in the group Sphaeroceratinae of some small-winged or completely wing-less species (of the genera Aptilotus, Apterina, Speomyza, some Limosina), the presence of which [in the present group of which the winged forms belong to the Sphaeroceratoid subtype] together with the high degree of specialization of the wings in this subtype, forbid to take sphaeroceratoid wings to represent a progressive direction of evolution of the muscoid type. This subtype, undoubtedly, is a good example of specialization, determined by the working out of a peculiar flight with high wing-beat frequency (to which bears witness the costalization).
The source of the sphaeroceratoid subtype, apparently, were forms of anthomyioid wings, in which disconnections in the Costal vein began to develop, [and in which began] to increase the anal lobe and alula, together with the slowed-down processes of perfection of the skeleton of the very wing-blade, having preserved a large intermedial cell. In more detail clarifying the causes of the realization of this line of evolution is now [still] not possible.
12. Tachinoid subtype.
The diverse diptera, belonging to the families Larvaevoridae (subfamilies Sarcophaginae -- first two images of next Figure, Oestrinae, some Larvaevorinae -- bottom image of next Figure and top image of next-next Figure, Melanophorinae -- middle image of next-next Figure, and Dexiinae), Cylindromyiidae -- last image of next-next Figure, Hypodermatidae, and Glossinidae -- Figure 38 , - together make up the special characteristic tachinoid subtype (from the name of the genus Tachina Meig., a well known representative of the tachinid flies, Larvaevoridae).

Figure 36 : Lifting lightly-veined wings of representatives of the tachinoid subtype of the muscoid type.
Top image : Taxigramma hilarella Zett., Larvaevoridae [Larvaevoridae s.str. = Tachinidae]. Length 3.75 mm.
(After ROHDENDORF, 1951).
Middle image : Nyctella egregia Zim., Larvaevoridae. Length about 2 mm. (After ZIMIN).
Bottom image : Linnaemyia vulpina Fall., Larvaevoridae. Length 8 mm. (After RODENDORF, 1951).
[Regarding Nyctella (middle image) it is interesting to note that we here have a very small wing in which in fact the complete muscoid venation is preserved -- something we would expect in large, not in minute wings. Indeed, we here see, the 2-branched Radial Sector, the curved anterior Medial vein, the posterior Medial vein, the intermedial cell, the posterior basal cell, and the cubital cell. And this whole venational network has been scaled down and in its entirety shifted towards the basal anterior part of the wing, leaving a large vein-free field in the posterior-apical half of the wing-blade. Indeed, this is a remarkable 'method' of costalization.]
(Images from ROHDENDORF, 1951)

Figure 37 : Some more lifting lightly-veined wings of representatives of the tachinoid subtype of the muscoid type.
Top image : Voria trepida Meig., Larvaevoridae. Length 6 mm. (After ROHDENDORF, 1951).
Middle image : Melanophora roralis L., Larvaevoridae. Length about 4.5 mm. (After LUNDBECK).
Bottom image : Cylindromyia brassicaria L., Cylindromyiidae. Length about 8 mm. (After LUNDBECK).
(From ROHDENDORF, 1951)

Figure 38 : One more representative of the tachinoid subtype of the lifting lightly-veined (muscoid) functional wing-type.
Glossina palpalis (tsetse fly). Female. Family Glossinidae. About 1 cm.
(From RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977)
Most characteristic of tachinoid wings is the development of a wide vein-free area in the posterior part of the wing, together with the presence of the large 5th radial cell. All veins of the wing are strong with about uniform thickness (apart from their basal sections). The Costal vein is strong, provided with three clear disconnections or thinnings : before the humeral cross-vein, directly after it, and directly before the inflow of the Subcostal vein (which vein is always well expressed). The Costal vein is, moreover, covered with strong lying bristles, of which some are sometimes larger than others, projecting. All three Radial branches end up at the anterior margin of the wing. The anterior one is the strongest, sometimes provided with bristles, it is the shortest. The middle branch is long, usually close to the posterior branch, which is the longest. The anterior Medial vein almost always with a sharp bend or kink, forming the so-called apical cross-vein (anterior branch). Sometimes at the place of the bend of the vein a fold or outgrowth (rudiment of the posterior branchlet) is present. Sometimes the end of the anterior Medial branch does not form a bend and only markedly curves upward [towards the posterior Radial vein] (Glossinidae -- Figure 38 , and Hypodermatidae). The anterior branch ends up in the Costal vein at the place of its end or in the last Radial vein. In that case the 5th radial cell is closed or even "petiolate" (see Figure 37 last two images). This characteristic fifth radial cell, similar to that in the muscoid-proper subtype, differs by its relatively small size. Comparing its width with that of the vein-free area of the hind-part of the wing, we must note that in the representatives of the tachinoid subtype this cell is either equal, as to its width, to the mentioned vein-free area or narrower than it, sometimes very significantly so (for instance three, four, and more times in some genera of Metopiini). The mentioned feature is the most neat and clear difference between these two related subtypes of muscoid wings. The intermedial cell is almost always present, large. The cells in the basal part of the wing are always well developed, large. Anal lobe, alula, thoracic- and wing-scale well developed, very large. Absolute size [of the insects] medium or large, ranging from 3 mm (some Metopiini, for instance species of the genera Nyctella and Apodacra) to 20 mm (some Larvaevorinae, for instance species of the genus Tachina). Usually the sizes are equal to 5-12 mm. Relative size of the wings not large, they usually are markedly shorter than the body. Magnitudes of surface area and load of the wings are investigated only in some Larvaevoridae, namely in representatives of the subfamilies Larvaevorinae and Sarcophaginae. Thus, in representatives of the genera Tachina, Larvaevora, Voria (Larvaevorinae) the surface area [always of both wings taken together] is equal to 0.176-0.624 cm2, load equal to 0.080-0.147 gr/cm2. In the genera Taxigramma, Bellieria, Sarcophaga and Coprosarcophaga the surface area is equal to 0.070-0.600 cm2, load equal to 0.057-0.177 gr/cm2.
13. Gastrophiloid subtype.
One small, poor in species, group of parasitic diptera, namely the family of intestinal warble flies, Gastrophilidae (next Figure), possess such peculiar wings forcing us to establish the special gastrophiloid subtype.

Figure 39 : Wings of the gastrophiloid subtype of the lifting lightly-veined (muscoid) functional type.
Top image : Gastrophilus intestinalis Deg., Gastrophilidae. Length 9 mm.
Bottom image : Gastrophilus haemorrhoidalis L., Gastrophilidae. Length 7.5 mm.
(After ROHDENDORF, 1951)

Figure 40 : Gastrophilus intestinalis Deg., Gasterophilidae. 14 mm. (gastrophiloid subtype of muscoid wings).
(After CHINERY, in Elseviers insektengids voor West-Europa, 1983)
The most characteristic features of this subtype are the shift of the Radial veins [towards the anterior wing-margin] and the weakening of the intermedial cross-vein, a general costalization of the venation together with the absence of the bend of the anterior Medial vein. The Costal vein is almost completely straight, strong up to the end of the last Radial vein and, while becoming gradually markedly weaker, reaching the wing-apex. Disconnections are absent in it. There only are well expressed transparencies at the corresponding places. The Subcostal vein is weak, but complete and well visible, ending up into the Costa. All three Radial veins run closely to each other, long and almost straight. The posterior Radial vein often weak and pale (G. haemorrhoidalis L., Figure 39, bottom image). The anterior Medial vein almost completely straight, a little curved down, sharply diverging from the last Radial vein, ending up at the wing's hind-margin far from the apex. The posterior Medial vein still more curving down. The intermedial cell, as a result of the shift of the intermedial cross-vein, very small, equal or shorter than the anterior basal cell [meant is -- I suppose -- the posterior basal cell, i.e. the cell directly basad of the intermedial cell and above the cubital cell]. Medial veins weak, often almost in the form of mere traces or folds. The posterior basal cell and the cubital cell well individualized, large. Both anal veins clearly present, where the first reaches the wing-margin. The central cell in the basiala is large. Anal lobe and alula very large. Small, but well developed wing- and thoracic scales are present. The posterior marginal area of the wing consists of large areas of the membrane free of veins and containing longitudinal folds and wrinkles. Microtricha, covering almost the whole wing-membrane, are absent in certain places of the cells of the basal part of the wing. Absolute body-size of the insects is large -- from 10 to 16 mm. The wings are a little shorter than the body. Magnitudes of surface area [always of both wings taken together] and load is determined in two species of the genus Gastrophilus : In G. intestinalis Deg. they are equal to 0.455-0.606 cm2 and 0.134-0.214 gr/cm2, in G. haemorrhoidalis L. to 0.385-0.461 cm2 and 0.126-0.180 gr/cm2.
As to the size and weight differences between the representatives of the two subtypes Rohdendorf further remarks :
Recall, that the linear sizes of the mining-flies (Agromyzidae) vary from 1 to 4 mm, and those of the intestinal warble-flies [Gastrophilidae] from 10-16 mm, that is, on average the body length of the former is equal to 2.5 mm, and of the latter 13 mm. Realizing that, roughly, the relation between body-weights being equal to the relation between the cubes of their linear sizes, we see that Gastrophilidae are on average about 140 times heavier than Agromyzidae ( 133 : 2.53 = 140.6 ).
The mentioned differences between gastrophiloid wings and agromyzoid wings give us a clue for understanding functional features. First of all [in gastrophilids, Figure 39] the perfect shape of the wing as a fastly and strongly beating organ is characteristic, i.e. the wing with its straight and firm anterior edge and its small apex. Not less characteristic is the structure of the wing-membrane [still in gastrophilids] provided with folds and wrinkles, that is, with a peculiar fan [corrugated sheet], having important aerodynamic significance, and, moreover, considerably increasing the firmness of the membrane. These features are completely absent in the wings of the agromyzoid subtype (Figure 29 ), having a different shape with a more or less convex anterior wing-edge and [with] disconnections in the Costal vein. The membrane of these minute and most minute wings is always devoid of a wrinkled fan.
The establishment of the differences of the features of the mentioned two subtypes, which bear witness to different aerodynamics [i.e. different flight-regimes], of course still is far from elucidating the similarity of the wing-skeleton and the courses of its evolution. For clarification of these questions it is necessary to take into account the phylogenetic connections of the Gastrophilidae, the life-history of these insects, the courses of the formation of their characteristic parasitism, and the ways of reproduction. Dealing with all these aspects of the problem would lead us far beyond the limits of the present investigation and cannot completely dealt with here. It is necessary only to underline that the development of larvae in conditions of abundance of food (in the body of a mammal) and the necessity of fast and governable flight (as a result of chasing fast-running ungulates-hosts to deposit eggs in their wool) determined the increase of body-size of these flies. This very probable process of increase of size of the warble-flies [here, Gastrophilidae] in their evolution seems to me a very important elucidating moment for the understanding of the formation of their flight-organs. Original forms of the warble-flies, not yet having reached the large sizes of the recent species, must have had already possessed a simple [i.e. not branched] straight anterior Medial vein, devoid of an anterior branch, that is, the costalization of the wings of the minute ancestors of the warble-flies must have had reached the condition that we observe in representatives of the anthomyioid subtype. Increase of size in intestinal warble-flies is therefore a comparatively new, recent, process. To this bears witness the absence of the anterior branch of the Medial vein (the so-called apical cross-vein), that would necessarily be preserved as a useful skeletal element of the wing-blade in the case of the former separation of the Gastrophilidae from ancestors [not with anthomyioid, but] with clythioid, muscoid-proper, or tachinoid wings [because then the anterior branchlet of the [anterior] Medial vein would have been preserved].
Concluding this concise analysis of the features of the gastrophiloid subtype, we must say that it is a derivative of one or another form of the anthomyioid subtype, mechanically having worked out the perfect wings of large flies on the basis of sufficiently costalized wings of their closest ancestors. I abstain from further analyzing the life-history of these insects because it is well known : winged Gastrophildae, being in their larval stage internal parasites of horses and allies, spend a large part of their active life in flight. From here it is clear, on the one hand, the evaluation of this subtype as being a progressive direction of the evolution of muscoid wings, and, on the other hand, the evident conditionality of this determination referring to only a single system of organs, in the present case the flight-organs. There is also no basis to reckon the group Gastrophilidae as a whole to represent a progressive evolution -- the narrow specialization of these parasites is beyond doubt. [Progressive evolution means an increase of potentialities to later occupy a diversity of new and different ecological niches, whereas narrow specialization is apt to become an evolutionary end-point from which fanning out to other ecological niches is not possible anymore. Regressive evolution is close to narrow specialization, or is a way to extinction.]
14. Hippoboscoid subtype.
The special parasitic diptera, the blood-suckers, Hippoboscidae, possess wings making up the characteristic hippoboscoid subtype (which is the last subtype of the lifting lightly-veined (muscoid) functional type).
To begin with, let us give some Figures.

Figure 41 : Lifting lightly-veined (muscoid) wings of representatives of the hippoboscoid subtype.
Top image : Wing of Lipoptena cervi L., Hippoboscidae. Length 5 mm.
Middle image : Wing of Hippobosca camelina Lch., Hippoboscidae. Length 10 mm.
Bottom image : Wing of Ornitheza sp., Hippoboscidae. Length 4 mm.
(After ROHDENDORF, 1951)

Figure 42 : Two common African louse-flies (Hippoboscidae), parasitizing on birds.
Ornithoctona laticornis (top image) possesses well developed wings, whereas they are strongly reduced in Crataerina [Crataerhina] acutipennis (bottom image), a parasite of the swift. 6 mm.
(After SKAIFE, LEDGER, and BANNISTER, Afrikanische insekten, 1981 (German translation of African Insect Life, 1953))
Most characteristic of this subtype is the development of extreme costalization, expressed by the shift of the whole venational network towards the anterior wing-margin, [resulting in] the large vein-free area of the wing-blade. The Costal vein is far rom reaching the wing-apex. It is strong up to the point of outflow of the last, third, Radial branch. In the basal section of the Costal vein there are transparences or even disconnections. Sometimes the basal section of the Costal vein almost completely disappears and becomes a very thin delicate edge of the wing-membrane (genus Lipoptena, Figure 41, top image). There are three or two (Lipoptena) Radial veins, strong, and running closely to each other. Medial veins straight, thin (being strong only in their basal parts), usually not reaching wing-margin, sometimes completely reduced as to their distal halves. Only the radio-medial cross-vein is always present. The intermedial cross-vein strongly shifted towards the wing-base, sometimes absent (genus Lynchia). The cubital cell is absent as a result of the reduction of the end of the Cubital vein -- genera Hippobosca (Figure 41, middle image), Lipoptena, Lynchia, and others. A large part of the wing-blade is devoid of veins, whereby the membrane is often strengthened by longitudinal wrinkles, that is, by a special fine corrugation of it (genus Hippobosca). Alula and scales usually absent, more rarely present -- genera Hippobosca, Ornitheza (Figure 41, bottom image), and Ornithomyia. Sometimes the wings are significantly narrowed as a result of the reduction of the vein-free membrane of the posterior marginal area (genera Stenopteryx, Crataerhina [Figure 42, bottom image] ). Data of surface area and load are absent.
End of the description of the fourteen subtypes of muscoid wings.
What follows is a summary of the origins of the subtypes of muscoid wings from one another (still using ROHDENDORF's text (pp.137), and adding comments and extra data). [And after that -- in the next documents -- we will continue with the remaining functional types of dipterous wings]
The listing of the features and diversity of the muscoid type of wings indicated the presence in the recent fauna of diptera of not less than 14 fairly clearly individualized secondary divisions of the type, its subtypes.
|
 (After ROHDENDORF, 1951, with translations) |
Figure 43 : Scheme of the interrelationships of the lifting lightly-veined (muscoid) functional wing-type with other types (XII and XV), and within it [of the interrationships] of its subtypes.
The figures indicating types and subtypes are spatially distributed in the scheme according to provisionally taken coordinates [of the space qualitatively defined by the specifications of the two dimensions (arrows) ].
Symbols : Roman numerals indicate types, namely XII - lifting many-veined (empidoid) type. XV - traction-lifting lightly-veined (syrphoid) type [whereas the present type, the lifting lightly-veined (muscoid) type, has the number XVI ].
The arabic numerals indicate subtypes according to the added table.
Summarizing all what has been said above, we see that the source of the muscoid type were microphoroid wings of the empidoid type [see Part LXXIII and next Figure] :

Figure 44 : Venation of Microphorus velutinus (Empididae). Microphoroid subtype of empidoid type.
on the basis of which the two first subtypes of the muscoid wings were formed -- dolichopodoid and clythioid wings.
It is now possible to consider the features of only two superfamilies of myiomorphs [i.e. of the infraorder Myiomorpha, consisting of superfamilies of higher flies, such as acalyptrates and calyptrates], containing parasitic flies -- intestinal warble-flies (intestinal gadflies), Gastrophilidea, and the blood-sucker flies, Hippoboscidea. The gastrophilideans are "warble flies" -- their larvae live in the tissues and intestinal tract of mammals, whereas the winged forms are aphagous [i.e. they do not take food], their mouth-parts being always more or less reduced. The organization of these diptera points to the absence of direct phylogenetic connections with other groups of warble-flies which are members of the superfamily Oestridea. On the other hand, a number of features (structure of the wing-venation, scales, head) clearly point to a relationship of the gastrophilideans with acalyptrate flies, which, apparently, form their ancestral group. The large size of the intestinal warble-flies (Gastrophilidae), as was already noted by me earlier (Rohdendorf, 1951, p.135-136), unconditionally is a recent acquisition in their history. The features of reproduction, in fact uncovered with sufficient completeness only in the most recent times by Soviet investigators (Tsjerjeschnev, 1951, 1953), point to a connection of these parasitic insects with the vegetation, with the grassy covering, which is totally unusual in warble-flies and together with this confirms the point of view about the connection of these flies with the acalyptrates. The nature of the solution of the [biological-physiological] conflicts, having determined the origin of the gastrophilideans, is thought to be as follows : The original minute forms had free-living larvae, living on the surface of plants or of the soil, consuming initially some sort of protein-containing matter (remains of dead bodies, or excrements of vertebrates, polluting the grassy vegetation or the soil). A frequently ending up [of the larvae] together with the food in the mouth of ungulates [such as horses or cows], the absence of principal biochemical differences between the protein media of these conditions (mucus, blood, or liquids [oozing] from carrion, on the one hand, and the excretion of the slimy film [mucous membrane] of the mouth of the animal, on the other) -- all this allowed to work out a firm biological connection between the dipterous larvae and the ungulate mammal. It is understood why the gastrophilids did establish connections with odd-toed ungulates (such as horses) and not with ruminants (such a cows). Intake of food by ruminants may be characterized as a relatively very rapid swallowing of torn off plant material, without preliminary intensive grinding, wherby in the mouth-cavity fresh plant material remains only for a short while, and having entered the stomach is quickly subjected to the action of digestive saps, unconditionally very alien and harmful to the free-living or connected with a protein medium dipterous larvae. Completely different things we see in odd-toed ungulates [perissodactyls] where the phenomenon of rumination [(re)chewing the cud] is absent. Torn off plant material undergoes an intensive grinding and remains in the mouth-cavity unconditionally for a longer time. The most-minute larvae, having ended up in the mouth-cavity of an odd-toed ungulate and washed away by saliva into the folds of mucous membrane in the mouth-cavity, do have more time to adjust to their new environment not having such unwholesome properties as does have the content of the stomachs of ruminants.
Summarizing what has been said about the courses of the historical development of the gastrophilids, we must note the evidently insignificant progressivity of these processes, having the nature of narrow specialization. The initial solution of conflicts was determined by the perfection of feeding, making it possible for the larvae to work out the ability to live in tissues and in the mouth-cavities of mammals. The further development of the gastrophilideans was determined by already clearly progressive processes -- perfection of individual development and reproduction. But the limitations of the mentioned main course of development (parasitism) could not be removed by the further progressive acquisitions which have taken place in the history of the gastrophilideans, and these diptera turned out to be in a position of a dying out relict-group, of which the further history is fully determined by the fate of their hosts, the dying out odd-toed ungulates.
There remains to consider two subtypes, also derivatives of anthomyioid wings, in which the processes of costalization have played a subordinated role. Such are the tyloid and drosophiloid wings.
In the tyloid subtype we have to do with special forms of narrow wings reminding us of the corynetoid subtype of empidoid wings, and, probably, having originated by the same reasons. The strong development of the legs, being the chief organs of locomotion [in representatives of the tyloid subtype, see Figure25 above], makes flight a subordinated auxiliary function of locomotion in these insects, living among dense grassy or shrubby vegetation or in tropical forest. These narrow tyloid wings cannot, apparently, execute fast wing-beats and only realize a little-governable flight of short duration -- a jump. Such a regressive nature of tyloid wings, is, in addition to processes of reduction of broadened membraneous structures at the posterior margin of the wing [for instance the non-development of an alula], also shown by the presence of short-winged not-flying insects that are direct derivatives of the forms of this subtype [i.e. they are taxonomically related].
An altogether different nature is shown in the formation of the drosophiloid subtype, the least transformed derivatives of anthomyioid wings. In this subtype body-size decreased, the relative size of the wings is rather large, processes of costalization are comparably weak and are expressed by the shift toward the wing-base of the intermedial cross-vein (this feature -- shortening of the intermedial cell -- most clearly distinguishes these forms from the anthomyioid subtype), finally, widely distributed in drosophiloid wings are processes of reduction of the cross-veins in the wing-base, leading to the vanishing of the posterior basal and cubital cells and to the development of disconnections in the Costal vein. Apparently, all these processes bear witness to a certain perfection of drosophiloid wings into the direction of the working-out of elastic and light flight-organs of minute insects, able to perform a slow flight with a relatively low wing-beat frequency. Locomotion of these insects on the substrate is not particularly fast, but the insects very often do it. The legs are adhesive [hooks], weakly armoured with bristles. The life-history of these flies is, as a rule, connected with lower plants, [with] mushrooms, and [with] yeasts. In the majority of forms development takes place in rotting or fermenting plant materials rich in yeasts. The multiformity of the taxonomic content of this subtype determines its great variability. Drosophiloid wings are possessed by representatives of not less than ten subfamilies of Drosophilidae and Lauxaniidae.
The last, the asteioid subtype, of muscoid wings is a characteristic derivative of the drosophiloid subtype. Asteioid wings (Figure 33) are characterized by their elongate shape, by the presence of only two long veins, weak development or complete reduction of lobes at the posterior wing-margin, and by a peculiar costalization. Overall body size small. Legs weak, adhesive [hooks]. The life-history is poorly known. It is known that they live in xerophilous habitats or amidst grassy vegetation. Individual development is known of a few forms and takes place in tissues of plants. All these traits of the subtype clearly point to a narrow and peculiar specialization, having gone along the way of increasing the wing's surface area realized by its elongation. Such a way of perfecting the flight-apparatus is rather rare in cyclorraphic flies and in fact may not be comparable to any other group. Only the dorilaoid subtype of syrphoid wings [yet to be considered] may partly be an analogue. To clarify in some degree the causes of the origin of the asteioid subtype is today [still] difficult and becomes possible only after a study of the living conditions of these insects.
( End of the exposition of the lifting lightly-veined (muscoid) type and all its subtypes)
Having concluded the exposition of the lifting lightly-veined (muscoid) functional wing-type, we will, in the documents to come, continue with the remaining wing-types in diptera. So, in the next document we will consider the lifting not-costalized (musidoroid) functional wing-type.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for further study of Organic Evolution, Part LXXV.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII
Back to Evolutionary Part XLVIII
Back to Evolutionary Part XLIX
Back to Evolutionary Part LIII
Back to Evolutionary Part LVII
Back to Evolutionary Part LVIII
Back to Evolutionary Part LXII
Back to Evolutionary Part LXIII
Back to Evolutionary Part LXIV
Back to Evolutionary Part LXVI
Back to Evolutionary Part LXVII
Back to Evolutionary Part LXVIII
Back to Evolutionary Part LXIX
Back to Evolutionary Part LXXa
Back to Evolutionary Part LXXb
Back to Evolutionary Part LXXI
Back to Evolutionary Part LXXI
Back to Evolutionary Part LXXII
Back to Evolutionary Part LXXIIa
Back to Evolutionary Part LXXIII
Back to Evolutionary Part LXXIIIa