Crystals grow in a melt (= molten mass) or in a solution [ NOTE 1 ], if certain conditions (in such a melt or in such a solution) are satisfied that relate to (1) the nature and concentration of the relevant atomic (ionic-, or molecular) species (concentration = the number of atomic (etc) individuals in a unit of volume), and further (2) the temperature and pressure (thermodynamic conditions).
These conditions accordingly can be such that crystallization sets in.
What kind of crystals appears, i.e. what structure actually appears, depends on more special conditions.
Crystals are composed of atoms and/or ions (electrically charged atoms) and/or ionic complexes and/or molecules. Naturally occurring crystals are called minerals. When they are allowed to grow freely they develop faces. Every such crystal is enclosed by a definite set of faces. The occurrence of faces points to a regular internal order. The internal order of crystals is based on an ordered atomic arrangement. This arrangement is moreover periodic. This means that a certain atom (or ion) is present at exactly the same structural (atomic) site throughout an essentially infinite atomic array. An atom at the same atomic site means that it is surrounded by an identical arrangement of neighboring atoms throughout the structure which consists of millions of unit cells (= the unit of structure which is repeated) with dimensions on the order of 5 to 20 Angstrom (1 Angstrom = 10-8 cm). A unit cell is in fact a basic spatial configuration of some geometrical points (configured into some basic forms like a cube or a parallelepiped). If it is repeated by continual translations (shifts) in three specific directions a point lattice will be formed which represents (i.e. describes) the periodic arrangement of the constituents in a crystal. We can think of the lattice points being occupied, or surrounded, by atoms (ions) or groups of them.
A crystal grows layer by layer by means of accepting certain constituents present in the growing environment (solution, melt or vapor), and in this way extends its lattice. However, the resulting internal periodic ordering (not directly visible macroscopically) is only perfect in 'ideal' crystals. In real crystals imperfections (defects) occur which locally disturb (geometrically) the regular arrangement of the crystal constituents. Such a geometrical disorder may consists in local dislocations. Another kind of imperfection can be caused by chemical zonation of a crystal.
The best way to better understand Crystals, including their nature as Totalities, consists in solving certain problems that pop up in considering them as such.
Substitution in Crystals
One of the problems is the phenomenon of substitution during crystal growth. Substitution does not refer to the replacement of previously (by the crystal) accepted atoms [ NOTE 2 ] by atoms of another species (which can happen at the surface of the growing crystal), but (substitution does refer) to the deposition (apposition) of atoms which do not belong to the presently crystallizing chemical compound [ NOTE 3 ] (which often is present in the solution in a dissociated condition), (a deposition) into places of the progressively extending crystal lattice, (places) which normally would be occupied by atoms of the original chemical compound (See previous Note). So the lattice points of the extending (growing) crystal are occupied by (or associated with) 'alien' atoms (atoms of another species) instead of atoms of the original chemical compound (See previous Note) (See the Essay Historical Individuum, Here-and-now Individuum, Section Metamorphosis).
However such a substitution phenomenon can only take place if the atomic diameters, or, more precisely expressed, the action radii, of the original atoms are almost equal to those of the alien atoms (We should keep in mind that the action radii of different atomic species are never exactly equal). Said differently, substitution is possible if the crystal lattice of the alien chemical compound differs not too much from that of the original compound. And because, as has been said, each atomic species differs in its action radius from any other atomic species, those lattices can never be exactly the same, but some could nevertheless be similar enough for, in the case of substitution, one crystal to be generated.
Such a substitution can take place in two ways :
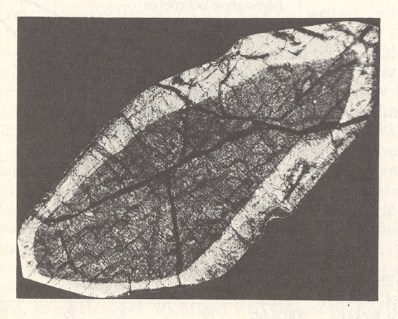
Figure 1. Zone structure in a crystal. Thin slice of a Pyroxene in polarized light.
In this case the crystal originated from a melt (molten mass), that has rapidly cooled off.
A Pyroxene is a kind of silicate mineral.
(After STRÜBEL, 1977)
What then is the nature of the Totality formed in this way? Is it still a true Totality, or has it perhaps become an aggregate ? And in the second case, should we, when substitution commences, speak of a substantial change, or of only a replacement of one state by another, while the one crystal unfolds? (This question is already discussed in the Section mentioned above on Metamorphosis).
R+C becomes R+C'
So at first the crystal grows according to S+C, and then according to S+C'. A S+C' process lays itself on top of a S+C process resulting in a zonated crystal. It is clear that this one zonated crystal is an aggregate. In this case an aggregate of two crystals (forming, in a way, one crystal). The degree of being an aggregate is in this case fairly weak, thus implying a situation of it somewhat removed from the aggregate side of the scale Aggregate ------------- Continuum.
The phenomenon of being a zonated crystal, and thus, in a way being one crystal, should be interpreted as just a phenotypical effect, and moreover a per accidens effect : Another Substance (also in the metaphysical sense) just happened to grow on top of the original one, together forming a certain unity because the respective lattices happened to be very similar. The respective Dynamical Laws, and thus the respective Essences, are different nontheless, and this is a genotypical state of affairs.
The second crystal, as it is present in the zonated crystal, has, it is true, the appearance of just a fragment of a crystal instead of a full-fledged crystal, enclosed within its faces, but even as such it is a genuine being, because the opportunity for a crystal to actually develop faces, especially a set of faces that neatly encloses the whole crystal, is per accidens. It depends on the surroundings of the growing crystal whether it can grow freely and develop faces, or not.
There are three reasons to interpret such a mixed crystal as an aggregate, despite the fact that we have to do with (numerically) one crystal. However, in this case not an aggregate of two crystals (thus not an aggregate at the crystal level) but an aggregate of atoms (an aggregate at the atomic level) :

Figure 2. Perthite, as a result of demixing.
Microperthitic demixing of high temperature mixed crystals with chemical composition
(K, Na)AlSi3O8 into Albite, NaAlSi3O8 (light) and Orthoclase, KAlSi3O8 (dark).
(After STRÜBEL, 1977)
A homogeneous mixed crystal (and also after its demixing by changed p/t - pressure and temperature - conditions) thus is (from a metaphysical point of view) an aggregate, and in the case of demixing this aggregate shifts still closer to the aggregate side of the scale (true) Aggregate ----------- (true) Continuum :
The homogeneous mixed crystal shows a stronger degree of unity than the corresponding demixed crystal. But this is a phenotypical state of affairs. In the genotypical domain, matters are clear and definite : The mixed crystal and the demixed crystal are each the product of ultimately two definitely specifically different Dynamical Laws (residing in the genotypical domain), and so such a crystal, mixed or demixed, represents two different species of Substance (in the metaphysical sense).
In the case of the homogeneous mixed crystal we should speak of an atomic mixture, an atomic aggregate, but, not at all just like that : The aggregate is organized into a lattice structure, albeit some sort of combination of two slightly different lattices. So as such it comes close to a (i.e. one) genuine being anyway, while its demixed form is just a set of several specifically different crystal individuals (depending on the composition of the original mixed crystal), loosely connected to each other. When seen in a bulky context (as in Figure 2), i.e. as a large set of individual crystals, and assuming that only two substances are involved, we can say that a subset of this set consists of (many) crystals of the one species, while the complement of this set consists of the other species of crystal. And of course all these constituent crystals together form just an aggregate, like we see in many natural rocks. So especially in the case of a homogeneous mixed crystal it is not easy to assess its ontological status, as either a (i.e. one) genuine being, or an aggregate.
A same chemical composition can accordingly be connected with different Space Groups (and even already with different Crystal Classes (Point Groups)), when we have to do with different p/t conditions.
So the compound Al2SiO5, Aluminum Silicate, occurs in three different mineral species (and thus in three different crystalline forms) :
| Mineral | Chemical Formula | Crystal Class (Point Group) | Space Group |
| Andalusite | Al2SiO5 | 2/m 2/m 2/m | P 21/n 21/n 2/m |
| Sillimanite | Al2SiO5 | 2/m 2/m 2/m | P 21/n 21/m 21/a |
| Disthene (= Kyanite) |
Al2SiO5 | 1* | P 1* |
(See STRÜBEL, 1977, p. 217)
Here we see the necessity of including the Space Group component in the per se description of a crystal. We also see that the mineral Aragonite (chemical composition : Calcium Carbonate) and the mineral Sillimanite (chemical composition : Aluminum Silicate) have the same Space Group (and even the same Point Group), so also the Chemical Composition component is indispensable for the per se description.
In chemically similar chemical compounds we still find differences in their crystal structure, even when the symmetry of the lattices is exactly identical. These differences consist in, for example, length proportions between the a-axis and the c-axis (STRÜBEL, 1977, p. 173) :
| Mineral | Chemical Formula | a : c | Crystal Class (Point Group) | Space Group |
| Calcite | CaCO3 | 1 : 0.854 | 3*2/m | R3*2/c |
| Rhodochrosite | MnCO3 | 1 : 0.818 | 3*2/m | R3*2/c |
| Siderite | FeCO3 | 1 : 0.814 | 3*2/m | R3*2/c |
| Smithsonite | ZnCO3 | 1 : 0.806 | 3*2/m | R3*2/c |
| Magnesite | MgCO3 | 1 : 0.811 | 3*2/m | R3*2/c |
Here we see the necessity of including the Chemical Composition component into the the per se description.
Chemical Composition + Space group reflects accordingly the Dynamical Law, which operated during the growth of the crystal.
The concept of Dynamical Law has been related to the the per se characterization of a crystal in the form of : Space Group + Chemical Composition.
However one has synthesized crystals, the so-called Pseudo Crystals, in which the 5-fold symmetry, 'forbidden' for crystals [ NOTE 10 ], nevertheless occurs (In organisms this 5-fold symmetry is wide-spread). This implies that the inner structure, resulting in them, is not periodic in its nature (STEWART & GOLUBITSKY, 1993, Fearful Symmetry, Is God a geometer? p. 95/6, and BALL, 1994, Designing the Molecular World, Chemistry at the Frontier, pp. 122).
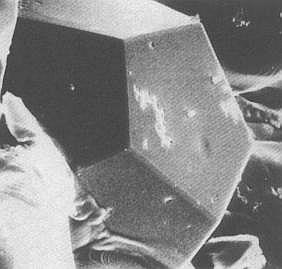
Figure 3. A Pseudo Crystal. These can have
shapes that reflect the forbidden symmetries
of the atomic structure. In this picture, the
pseudo crystal has a dodecahedral shape.
(After BALL, 1994)
This discovery could perhaps imply that the current Group Theoretical Paradigm (expressed with the concepts of Point Group and Space Group) is not necessarily all there is to say about crystal structure (STEWART & GOLUBITSKY, 1993, p. 96).
Looking again to the aforementioned system of Al2SiO5 we see that it is, from a chemical perspective, one system, which can give rise to several mineral phases : Andalusite, Sillimanite and Disthene. Each for themselves these phases are stable in a definite region of the Triple Point Diagram (STRÜBEL, 1977, p. 217) , which has two axes, the temperature axis and the pressure axis. So here we have the (one) system of Al2SiO5, with three mineral phases, and only at the Triple point all three mineral phases can co-exist. Of course not in a stable way.
Hoewever, within our Totality Consideration we have to do with three systems, because in such a consideration the crystals are central. Accordingly in this case we have to do with three Dynamical Laws (crystallization laws), and their respective domains of validity are given in the Triple Point Diagram.
Aggregates
The fact that minerals can form strange types of aggregates (or weak Totalities), can be illustrated by three examples, Opal, Wavellite and Rutilated Quartz.
Opal, SiO2 . n H2O
Although Opal is essentially amorphous, it was demonstrated that it nevertheless has an ordered structure. It is however not a crystal structure with atoms in a regular 3-dimensional array, but consists of closely packed silica spheres in hexagonal and / or cubic closest packing. Air or water occupy the spaces between the spheres.
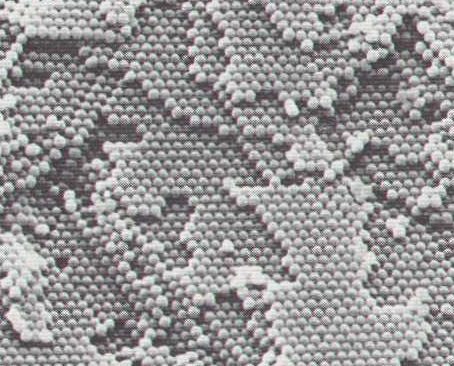
Figure 4. Scanning electron micrograph of an OPAL with chalky appearance showing hexagonal packing of silica spheres (diameter of spheres approximately 3000 Angstrom (1 Angstrom = 0.00000001 cm). Because of the weak bonding between the spheres they are completely intact. Here we have an example of an aggregate wich is almost a Totality, although the pattern is not very elaborate, it is just a dense packing of weakly bonded spheres, wich are themselves probably more stronger Totalities (consisting of SiO2 + Water).
( After Hurlbut & Klein, 1977, Manual of Mineralogy )
In ordinary Opal the regions of equally sized silica spheres with uniform packing are small or non-existent, but in precious Opal large regions are constituted of regularly packed spheres of the same size. The diameter of the spheres varies from one Opal sort to the other, and ranges from 1500 Angstrom to 3000 Angstrom (1 Angstrom = 10-8 cm) (HURLBUT & KLEIN, 1977, Manual of Mineralogy, p. 419).
Wavellite, Al3(PO4)2 (OH)3 . 5 H2O
Some minerals preferably form structured crystal aggregates, for example Wavellite (HURLBUT & KLEIN, 1977, p. 333 ; STRÜBEL, 1977, p. 343).
In the case of Wavellite individual crystals, i.e. free crystals, are rare. Usually it occurs in the form of radiating spherulitic and globular aggregates.

Figure 5. Wavellite from Dünsberg near Giessen (Germany)
(After STRÜBEL, 1977)
Wavellite crystallizes in the Orthorhombic Crystal System in the Crystal Class (= Point Group) 2/m 2/m 2/m, and has as Space Group Pcmn (abbreviated symbol).
Radiating crystal aggregates are also formed in Zeolites, for example Natrolite, and also in Limonite, Malachite and Antimonite.
Rutilated Quartz (Rutile, TiO2, Quartz, SiO2)
Also crystals within crystals do occur in Nature, for example Rutilated Quartz. This is Quartz which has fine needles of Rutile penetrating it.

Figure 6. Rutilated Quartz, Brazil
(After HURLBUT & KLEIN, 1977)
This we should interpret as an aggregate, although a more or less weak form of it. In this case it is an aggregate of two different crystal species, Quartz and Rutile.
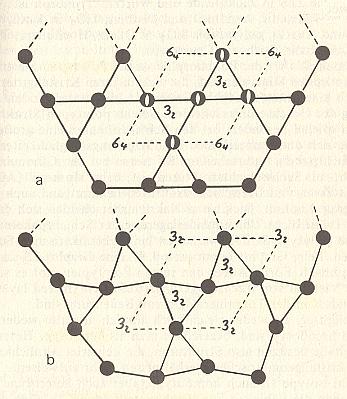
Figure 7. Lattices of low-Quartz (bottom) and high-Quartz (top).
Projection of the lattices in the direction of the c axis.
When paramorphosis occurs the outer form of high-Quartz is preserved after
its transformation into low-Quartz.
All the 6-fold screw axes ( 64 ) of high-Quartz become 3-fold screw axes ( 32 ) after
its transformation into low-Quartz.
The black disks represent Silicon ( Si ) atoms. The unit cell is outlined by dashed lines.
Its vertices are lattice points. Compare with Figure 9.
(After STRÜBEL, 1977)
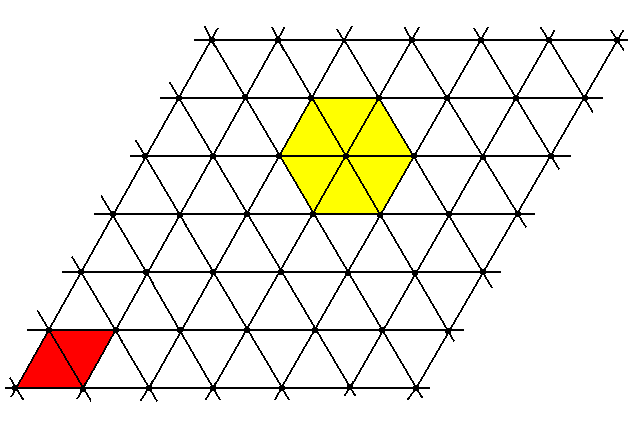
Figure 8. Two-dimensional hexagonal point lattice.
The nodes in the figure represent the points, and these in turn represent the centers of the packed spheres. The unit cell is a rhombus (red). The hexagonality is indicated by yellow coloring of a part of the lattice.
Something similar will be the case with the packing of atoms. And also a three-dimensional packing of them.
The crystal lattice represents the internal structure of a crystal. This structure is an ordered structure. In Nature there are many ordered structures. Some of these ordered structures are periodic, and it is this type of ordering that we encounter in crystals. It can be described with a three-dimensional lattice. A lattice is an abstract geometrical concept (as has been said, in order to describe the structure of a crystal). A lattice is accordingly a three-dimensional periodic ordering of points. In real crystals these lattice points can be occupied by atoms (ions, ion complexes or molecules). But such a coincidence of geometrical lattice points with (positions of) atoms is not compelling. Often they do not coincide. The lattice points are just geometrical points in the structure which all have the same angle and distance relations to the atoms that compose the crystal, and with it to the symmetry elements [ NOTE 12 ] of the crystal structure. Every lattice point is equivalent to every other lattice point. So every lattice point is associated with a same atomic pattern (sometimes this pattern consists of only one atom). The point lattice only indicates the way (i.e. the directions) of repetition of these atomic patterns, and also the distances between the repeated units. Surely each lattice point always represents the location of a motif, i.e. a pattern of atoms of which the geometrical center, or another representative point, coincides with a lattice point. If this motif consists of only one atom then the position of that atom coincides with the relevant lattice point. The geometrical lattice points reflect and represent accordingly the relevant periodic ordering of the crystal constituents (See KLEIN & HURLBUT, 1999, Manual of Mineralogy, p. 129, and especially the next figure).
Figure 9. A drawing of the structure of low (alpha) Quartz ( SiO2 ), with the normally vertical
z axis tilted at a small angle to better show the repeat distance
c (of the unit cell) along the
z direction. The primitive hexagonal space lattice [ NOTE 13 ] , outlined by the various parallelepipeds, shows that each unit cell (with edges a1 , a2 and c) contains a complete and representative unit of the repeating pattern of the structure. Large circles are Oxygen atoms (O), small circles are Silicon atoms ( Si ).
(After KLEIN & HURLBUT, 1999)
If we compare the above figure with Figure 8, then we clearly recognize the hexagonal arrangement of lattice points. We also see the chemical bonds and their directions (four bonds -valencies - for Silicon, two for Oxygen). Moreover it is clear from the picture that the point lattice is purely geometric in nature, describing the repetition of material units.
In most cases the points of the crystal lattice are, as has been said, each for themselves occupied by a pattern of several atoms. This pattern will then, in accordance with the geometry of the lattice, be repeated. The actual chemical structure of the crystal, thus the bonding of the individuals atoms to each other, forming for example SiO4 tetrahedrons [ NOTE 14 ], is not displayed by the lattice structure. The lattice structure only displays, as has been said, the nature of the periodic ordering of the crystal constituents. Of course there is a dependence relation between the chemical bonds on the one hand, and the lattice to be formed on the other. The next figure will make this clear.
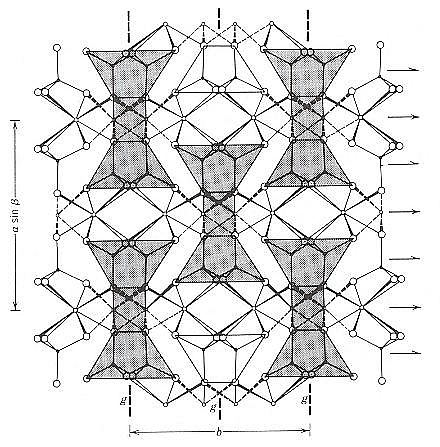
Figure 10. A view of the clinopyroxene structure along the c axis. The shaded parts identify repeats of some of the motifs in the structure. These structural units are repeated in a centered pattern in this view, i.e. four motifs at the corners and one in the center. In three dimensions this is compatible with a C-centered monoclinic lattice (denoted as C). So the geometrical centers of the motifs are the lattice points.
(Two types of) arrows indicate the location of 2-fold rotation axes and 2-fold screw axes - the latter as a result of the centering.
(After KLEIN & HURLBUT, 1999)
Also the next three figures, displaying the mineral Beryl, Be3Al2Si6O18, will show the relationship between the actual internal structure of a crystal of this mineral and the crystal lattice, describing the way of repetition of structural units.
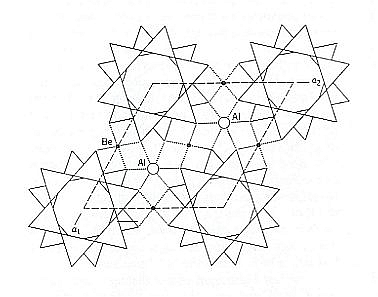
Figure 11. The structure of Beryl as projected onto (0001), i.e. projected onto the base face of the crystal. A unit cell is shown by dashed lines. The unique 6-fold Si6O18 rings are shown. In fact we see four pairs of such rings, each pair consisting of two rings (separated by other structural elements) in alternating orientation. Of course this whole structure keeps repeating itself.
If the centers of four of these rings are chosen as the positions for possible lattice nodes (points), the rhombus shape of the lattice becomes obvious, and this corresponds to a primitive hexagonal lattice choice. A two-dimensional version of such a lattice was pictured in figure 8.
If we repeat the unit cell of the Beryl structure several times, then we get the next Figure, in which the hexagonal arrangement becomes clear.
(After KLEIN & HURLBUT, 1999)
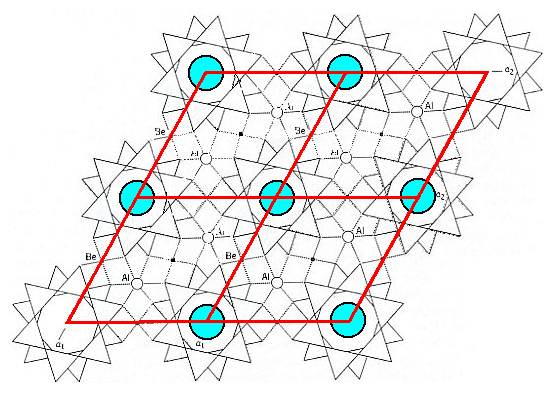
Figure 12. Structure of Beryl. Obtained by repeating the unit cell outlined in Figure 11, in order to show the hexagonal lattice.
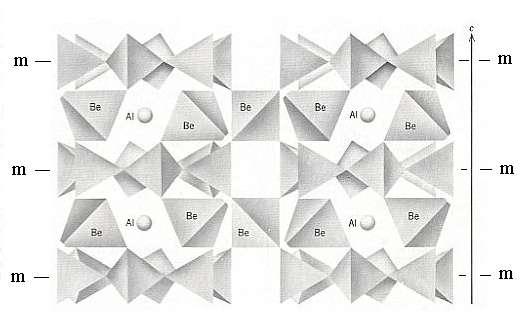
Figure 13. A vertical three-dimensional view of the Beryl structure. The location of horizontal mirrors through the centers of the rings is shown ( the hexagonal outline of, and horizontal mirrors through these rings account for 6/m in the Space Group symbol, which is P 6/m 2/c 2/c ). Be (Beryllium) and Al (Aluminum) provide cross-links between the Si6O18 rings.
(Adapted from KLEIN & HURLBUT, 1977)
Further it is important to realize that the presence of glide planes and screw axes [NOTE 15 ] often has the status of being introduced in virtue of the choice of a unit cell. In Space Groups with a lattice which (because of the choice of unit cell) is not primitive, i.e. with centering [ NOTE 16 ], screw axes and glide planes are introduced because of the centering. These new symmetry elements are not included in the Space Group symbols (because they are implied). So in the case of non-primitive lattice types one should be aware of this (KLEIN & HURLBUT, 1999, p. 139).
Because the Space Group is an essential part of the total effect of the Dynamical Law of the crystal (i.e. the total symmetry content of a crystal -- which is that essential part -- is generated by that particular Dynamical Law), we will elaborate a little more on the concept of Space Group, and of the related concept of Point Group.
The Point Group of a crystal is the translation-free residue of its Space Group, i.e. if we consider the total symmetry content of a crystal, and then eliminate all translational symmetry, then we end up with the Point Group of that crystal. Translational symmetry itself is not macroscopically visible, so what is left after its elimination is the macroscopically visible symmetry, expressed, among other things, by the shape of an undisturbed crystal (i.e. a crystal that grew freely under homogeneous conditions), the physical (and geometrical) equivalence between certain crystal faces, their arrangement with respect to certain directions (crystallographic axes), and so on. How do we mentally accomplish the just mentioned elimination of all translative symmetry? We can do this by mentally superimposing all motifs (atoms, or clusters thereof) of the lattice, resulting in one final motif. The (point) symmetry of this one motif then represents the point symmetry of the crystal, expressed by its Point Group.
The internal order causes the basic morphology of the crystal. Hence its Point Group can be derived from its Space Group.
So the Space Group governs the Point Group of the Crystal. It moreover governs the point symmetry of the individual motifs, and also the point symmetry of motif units, i.e. of parts of the motifs. Finally it governs the orientation of the motifs in the translational lattice, or, equivalently, it governs the orientation of the translational lattice with respect to the motifs which are placed in it. So the Space Group controls everything that has to do with the symmetry properties of a crystal. And, as has been said on many occasions, if we supplement the information about the Space Group to which a crystal belongs with information about its chemical composition, then the structure of the crystal is completely determined. This structure is the direct 'phenotypical' expression of the relevant Dynamical Law.
In all this we see that the structure of crystals is not a simple matter. In many cases we have to do with several lattices which are placed within each other, thus a structure in which several lattices penetrate each other. Let all this, as said, be complicated. Compared with Organisms Crystals are however very simple.
For a more extensive treatment of the internal structure of crystals (making much use of the two-dimensional analogues of (3-D) crystals), see the Essay on The Internal Structure of Crystals, Part I ---- Part XX.
In the next Essay of this Series, namely the Essay on The Morphology of Crystals, we will treat of the macroscopical features of Crystals, especially their macroscopic symmetry elements, constituting their Point Groups.